Preparation of 3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid
Nov 5,2019
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid(4-oxo-3-[(phenylmethyl)oxy]-4H-pyran-2-carboxylic acid, BPCA, 3-(benzyloxy)-4-oxo -4H-pyran-2-carboxylic acid) can be prepared according to the reported literatures[1-4].
Method 1
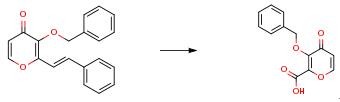
To a mixture of 822 g of the starting material (1.0 eq.) and 11.2 g of RuC13 nH2O (0.02 eq.) in 2.47 E of MeCN, 2.47 E of EtOAc and 2.47 E of H2O was added 2310 g of NaIO4 (4.0 eq.) at less 25 than 25°C. After aging for 1 h, 733 g of NaClO2 (3.0 eq.) was added to the mixture at less than 25° C. After aging for 1 h, precipitate was filtered and washed with 8.22 eq of EtOAc. To the filtrate, 1.64 eq of 50% Na2CO3 aq, 822 mg of H2O and 630 mg of HC1 were added. The 30 aqueous layer was extracted with 4.11 eq of EtOAc and the organic layers were combined and concentrated. To the residue, 4 eq of toluene was added and the mixture was concentrated and cooled with ice bath. Filtration, washing with 1 eq of toluene and drying provided 372 g of the product (56% yield) as a solid. 1H NMR (300 MHz, CDC13) ö 7.78 (d, J=5.7 Hz, 1H), 7.54-7.46 (m, 2H), 7.40-7.26 (m, 3H), 6.48 (d, J=5.7 Hz, 1H), 5.6 (brs, 1H), 5.31 (s, 2H).
Method 2

Add 2-(hydroxymethyl)-3-(benzyloxy)-4H-pyran-4-one to the reaction kettle 19.8 Kg, methylene chloride (176 L) and water (85 L) were stirred. Then add sodium bicarbonate (46Kg), sodium bromide (0.9Kg) and 2,2,6,6-tetramethylpiperidine -nitrogen-oxide (TEMPO) (1.4Kg), cooled to 5 °C, 10 percent sodium hypochlorite (175 kg) was added dropwise, and the mixture was stirred at 5 °C for 1 h, and the reaction was completed. A 30 percent aqueous sodium thiosulfate solution (119 L) was added and stirred for 1 h. Concentrated hydrochloric acid was adjusted to pH=3.5, the organic phase was separated, and the aqueous phase was dichloromethane (65L). The mixture was extracted twice, and the combined organic phases. The crude product was recrystallized from acetonitrile.3-(Benzyloxy)-4-oxo-4H-pyran-2- carboxylic acid 17.5 Kg. The purity was 99 percent and the yield was 83 percent.
Method 3

To a mixture of 10.0 g of the starting material and 13.6 mg of RuCI3 nH2O in 95 mL of MeCN and 10 mL of water, mixture of 155mL of water, 7.2 g of hydrosulfuric acid, and 15.5 g of Nalψ4was added for 2.5 h at 20 0C. After aging for 1 h, organic and aqueous layers were separated and aqueous layer was exracted by 30 mL of ethyl acetate. Aqueous layer was exracted again by 30 mL of ethyl acetate and organic layers were combined. 6 mL of 5 percent NaHSO3 solution was added to the combined organic layer and the layers were separated. The organic layer was adjusted to pH 6.0 by adding 4.0 g of 2M NaOH solution and the aqueous layer was separated. After 60 mL of 5 percent NaHCO3 solution and 257mg of TEMPO was added, 25.9 g of NaCIO solution was added to the reaction mixture at 25 0C for 1 h and stirred for 30 min to check the reaction was finished. After the layers were separated, 42.5mL of 5percent Na2SO3 solution and 30 mL of AcOEt were added and separated. The aqueous layer was exracted by 30 mL of AcOEt and separated. 12 percent H2SO4 was added to the reaction mixture at 20 0C for 1 h and the mixture was cooled to 5 0C. After the mixture was stirred for 30 min, the mixture was filtered, washed with 30 mL of water twice and dried to provide 5.7 g of the product (70 percent yield) as a crystal. 1H NMR(300 MHz, CDCI3) δ 7.78 (d, J = 5.7 Hz, 1H), 7.54-7.46 (m, 2H)1 7.40-7.26 (m, 3H), 6.48 (d, J = 5.7 Hz, 1H), 5.6 (brs, 1H)1 5.31 (s, 2H).
References
1. Shionogi & Co., Ltd., ViiV Healthcare Company. Johns BA, Weatherhead JG, Hakogi T, Aoyama Y. (3S,11aR)-6-[(phenylmethyl)oxy]-3-methyl-2,3,11,11a- tetrahydrooxazolo[3,2-a]pyrido[1,2-d]pyrazine-5,7-dione of the formula P-9 and/or (3S,11aR)-6-[(phenymethyl)oxy]-8-bromo-3-methyl-2,3,11,11a- tetrahydrooxazolo[3,2-a]pyrido[1,2-d]pyrazine-5,7-dione of the formula P-10. US9133216[P], 2015, B2, Page column 16.
2. Guangdong Laifoshi Pharmaceutical Co., Ltd. Ye W, Zhou Z, Fei A, Xie Y, Xi L. 3 - (benzyloxy) -4 - oxo - 4H - pyran -2 - carboxylic acid synthesizing method (by machine translation). CN109438405[P], 2019, A, Paragraph 0024; 0027; 0028; 0031; 0032; 0035; 0036; 0039.
3. GLAXOSMITHKLINE LLC; SHIONOGI &; CO., LTD. JOHNS BA, DUAN M, HAKOGI T. Processes and Intermediatesfor Carbamoylpyridone HIV Integrase Inhibitors. WO2010/68262[P], 2010, A1. Page column 22.
- Related articles
- Related Qustion
4,4-Difluoropiperidine hydrochloride is a heterocyclic fluorinated building block.....
Nov 5,2019Pyridine Compound2-Chloro-4-cyanopyridine is an important organic intermediate to synthetize substituted pyridines.....
Nov 5,2019Pyridine Compound3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid
119736-16-2You may like
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid manufacturers
- Baloxavir Marboxil Impurity 4
-

- $0.00 / 10mg
- 2024-04-28
- CAS:119736-16-2
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g
- Baloxavir Marboxil Impurity 4
-
- $0.00 / 10mg
- 2024-04-02
- CAS:119736-16-2
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g
- 3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid
-
- $0.00 / 25kg
- 2024-03-28
- CAS:119736-16-2
- Min. Order: 25kg
- Purity: 98%
- Supply Ability: Inquiry






