Propylene glycol: Applications, Toxicity and Synthesis
Apr 21,2023
Introduction
Propylene glycol[1], also known as 1,2-propanediol, is a synthetic organic compound with the chemical formula C3H8O2. It is a viscous, colorless, and odorless liquid that is soluble in water and many organic solvents. Propylene glycol is a diol, meaning it has two hydroxyl (OH) groups, which are responsible for its properties and applications. Propylene glycol has a wide range of uses, including as a solvent, humectant, emulsifier, and preservative in various industries. It is commonly used in the food, pharmaceutical, cosmetic, and industrial sectors. In the food industry, propylene glycol is used as a humectant, solvent, and preservative in numerous products, such as baked goods, dairy products, processed foods, and food additives. In the pharmaceutical industry, propylene glycol is used as a solvent and carrier for drugs administered orally or topically, and as a diluent for injectable drugs. It is also used as an ingredient in electronic cigarettes to deliver nicotine to users. In the cosmetic industry, propylene glycol is used as a humectant and solvent in lotions, creams, and other personal care products. It helps to retain moisture, and enhances the absorption of other ingredients. In the industrial sector, propylene glycol is used as a coolant and antifreeze in heating and cooling systems, as well as a solvent in the production of polyester resins, lubricants, and hydraulic fluids. Propylene glycol has low toxicity, and is generally considered safe when used in accordance with regulations. However, prolonged or excessive exposure can cause irritation to the eyes, skin, and respiratory system, especially in sensitive individuals. Therefore, it is important to handle and use propylene glycol properly to ensure safety.

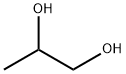
Figure 1 appearance of Propylene glycol
Application
Propylene glycol is a versatile organic compound with numerous applications across several industries. One of the primary uses of propylene glycol is as a solvent and humectant in the cosmetics and personal care industry. It is commonly found in products such as shampoos, lotions, and deodorants due to its ability to retain moisture and prevent drying. It is also used as a food additive in the production of processed foods and beverages. It is used as a humectant to keep foods moist, as well as a carrier for flavors and colors. In the pharmaceutical industry, propylene glycol is used as a solvent for various drugs and medications. It is also used as a preservative in some vaccines and medicines. Beyond these industries, it is also used as a coolant and antifreeze in the automotive and aerospace industries. It is also used as a de-icing agent on airplanes and runways during winter months.
Overall, propylene glycol has a wide range of applications due to its versatility and unique properties. Its ability to act as a solvent, humectant, and preservative makes it an essential ingredient in many consumer products across several industries[2].
Toxicity
Propylene glycol (PG) is generally considered safe for use in a variety of applications, including food, cosmetics, and pharmaceuticals. However, like many substances, it can be toxic at high enough doses. The toxicity of propylene glycol depends on the amount ingested or absorbed by the body. Ingesting large amounts of propylene glycol can cause symptoms such as headache, dizziness, confusion, lightheadedness, nausea, vomiting, and seizures. In rare cases, ingestion of very large amounts may cause coma or even death. In addition, some people may develop an allergic reaction to propylene glycol, which can cause symptoms such as hives, itching, swelling, and difficulty breathing. It's worth noting that propylene glycol is often used as a solvent in e-cigarettes and vaping liquids. While it is generally considered safe for inhalation in small amounts, inhaling large amounts of propylene glycol vapor can cause respiratory irritation and may exacerbate asthma and other respiratory conditions. Therefore, it is important to use caution when using products containing propylene glycol and to follow any safety guidelines provided by the manufacturer[3].
Synthesis
Using γ-AI203 as the carrier, the glycerol hydrolysis Cu-based catalyst was prepared by impregnation method. When the Cu load is 10%, the catalytic performance is better. Feed n(hydrogen): n(glycerol) = 30:1, 185℃, 3.0MPa; The conversion rate of glycerol was 94.62%, and the selection rate of 1.2-propanediol was 94.58%. The carrier was modified by Zr02, B203 or silicotungtic acid, which could adjust the acidity and alkalinity of the catalyst surface to prevent the aggregation of Cu species, improve its dispersibility, and improve its catalytic activity. The 10% Cu, 5% B203/ γ-Al203 catalyst prepared after B203 modification was prepared under the mass fraction of glycerol aqueous solution 30.0%, feed n (hydrogen): n (glycerol) = 30∶1, 185 ℃ and 3.0MPa. The conversion rate of glycerol reached 96.54%, and the selection rate of Propylene glycol was 96.65%. For 10% Cu 5% B203/γ-AI203 catalyst, the suitable reaction conditions for the hydrogenation of glycerol to synthesize Propylene glycol were temperature of 185 ℃, 3.0 MPa, and the ratio of raw material H2 to glycerol was 20. The concentration of glycerol aqueous solution increased, the conversion rate of glycerol decreased, the selection rate of Propylene glycol changed little, and the selection rate of ethylene glycol increased slightly[4]
References
[1] Li X, Liang YJ. Research progress on in the synthesis of Propylene glycol by in situ hydrogenation of glycerol[J].Science & Technology Vision,2019,(22):166+138.
[2] Gram-Negative Bacteria - Salmonella; Studies from Iowa State University Provide New Data on Salmonella (Engineering the Pdut Shell Protein To Modify the Permeability O the Propylene glycol Microcompartment of Salmonella)[J]. News of Science,2020.
[3] Sheng YH, Feng HM, Hu Y,et al. Experimental study on in vitro and in vitro safety of 1,2-propanediol[J].Modern Chinese Journal of Applied Pharmacy,2019,36(19):2391-2396.
[4] Li Chen, Wang Peili. Cu/γ-Al2O3 catalyzes the hydrogenolysis of glycerol into Propylene glycol [J].Journal of Henan University of Technology (Natural Science Edition),2016,37(01):100-103.
- Related articles
- Related Qustion
- Toxicity and human health effects of Propylene glycol Apr 18, 2024
Propylene glycol (PG) is a synthetic liquid that is commonly used in the food, pharmaceutical, and cosmetics industries. It serves as a solvent, humectant, and preservative.
- Applications of Propylene glycol Nov 7, 2019
Propylene glycol is a synthetic, colorless, odorless, tasteless liquid that belongs to the same chemical class as alcohol.
Methyl salicylate, also known as wintergreen oil, is a colorless to pale yellow liquid. It has a characteristic odor of wintergreen and is commonly used as a flavoring agent in the food industry.....
Apr 21,2023APITetrakis(triphenylphosphine)palladium is used in cross-coupling reactions such as the Suzuki coupling reaction and the Heck reaction. The compound is air-stable and soluble in organic solvents.....
Apr 21,2023APIPropylene glycol
57-55-6You may like
Propylene glycol manufacturers
- Propylene glycol
-

- $1.00 / 1g
- 2024-05-28
- CAS:57-55-6
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- 1,2-Propanediol
-

- $6.00 / 1kg
- 2024-05-18
- CAS:57-55-6
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Propylene glycol
-

- $1.70/ kg
- 2024-05-13
- CAS:57-55-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT