Synthesis and Application of Salcaprozate sodium
Nov 15,2022
General description
Salcaprozate Sodium, abbreviated as SNAC, is a bisocarbon phosphate compound absorption enhancer used in the treatment of gastrointestinal diseases, especially for bisocarbon phosphate compound malabsorption caused by gastrointestinal diseases.

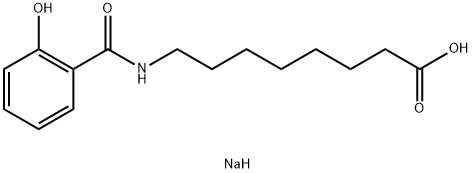
Fig. 1 The structure of Salcaprozate Sodium.
Synthetic routes

Fig. 2 The synthetic method 1 of Salcaprozate Sodium.
Dissolve 8-aminooctanoic acid in methanol containing thionyl chloride. Stir the mixture overnight. Add triethylamine dissolved into dichloromethane to the mixture. Add acetylsalicylate dissolved into DCM containing oxalyl chloride and triethylamine to the mixture. Dissolve the above products into methanol. Add the mixture to sodium hydroxide solution. Hydrolyze the ester. Dissolve the mixture into ethanol. Add sodium hydroxide solution accurately to the mixture [1].

Fig. 3 The synthetic step 1 of method 2 of Salcaprozate Sodium.
A dry, clean, 500 ml half jacketed, 4-neck round bottom flask, equipped with a mechanic stirrer, thermo couple, chiller, and an addition funnel, was charged with 153 g of water, 22 mg of EDTA (0.08 mmol), and 30 g of 2,4-dioxo-1,3-benzoazinyl-octanoic acid ethyl ester (76.49 mmol). The mixture was stirred for 30 minutes at 20 ± 5 °C. Then 29.22 g of 40% NaOH solution (292.18 mmol) was added to the mixture. The mixture was heated to ~97 °C and held for 20 hours. The mixture was then cooled to 20 ± 5 °C. The batch was charged to an addition funnel, and the flask was charged with 29 g of acetone and 36.25 g of 31% HCl. The batch in the first flask was transferred to the acetone/HCl solution over 40 minutes while maintaining temperature <30 °C. After the transfer, the pH of the batch was adjusted to ~4.5 with 20% NaOH solution. The mixture was heated to ~60 °C, held for 0.5 h, and then cooled to 20 ± 5 °C. The batch was held at 20 ± 5 °C for at least 2 hours. The solid was filtered, washed with water, and dried at 80 ± 5 °C under vacuum overnight to give 20.4 g (95% yield) of SNAC free acid [2].

Fig. 4 The synthetic step 2 of method 2 of Salcaprozate Sodium.
A 1L, half jacketed, 4-neck round bottom flask equipped with a mechanical stirrer, a thermo couple, an additional funnel, and a condenser, was charged with 46.35 g of SNAC free acid (165.9 mmol), and 180 ml of iPrOH and stirred at room temperature (rt). The suspension was heated to 40 °C. To the resulting suspension was added 33.84 g of 20% NaOH (169.2 mmol) solution over a span of 30 minutes. The suspension has become a clear solution when about only half of the base was added. After the full amount of base was added, the clear solution was at pH = 9.0. The reaction temperature was then raised to 50 °C and was stirred at 50 °C for 30 minutes. The almost colorless clear solution was cooled to 35 °C in one hour. The clear solution was then seeded with 100 mg of crystalline SNAC sodium salt (0.33 mol) and stirred at 35 °C for one hour. The clear solution has become a milky light suspension. The suspension was further cooled to 30 °C in one hour and hold at 30 °C for one hour, it has become a very thick white suspension. 180 ml of i-PrOH was added over a span of one hour. The internal temperature was kept at 30 °C through out the addition. The stirring actually became easier after the addition. The suspension was then cooled to 0 °C in a span of one hour and was aged at that temperature for 18 hours. The solid was filtered on a coarse sintered glass funnel and filtration was very fast. The solid was air-dried for one hour. The resulting white solid was transferred to a crystallization dish and was dried at 35 °C for 6 hours and at 90 °C with nitrogen bleeding for additional 18 hours. It was cooled in an oven to rt under vacuum (needs to be less than 40 °C) before removal from the oven. In total 46.8 g (93.6% yield) white solid was collected, which was found to by anhydrous SNAC sodium salt, with a monomodal particle size distribution. The water content was found to be 0.52%, as judged by Karl Fischer titration. The aqueous solution of the salt has pH = 7.0. Water content needs to be carefully monitored during drying process to make sure the water level is below 1%, preferably below 0.5%. Before filtration, the reaction content may be aged at 0 °C overnight. No quality deterioration was observed. The anhydrous monomodal SNAC sodium salt product has good solubility in water, significantly higher than that of a trihydrate form [2].
Application
Characterization of the physicochemical interactions
A common approach to tackle the poor intestinal membrane permeability of peptides after oral administration is to formulate them with a permeation enhancer (PE). Increased oral bioavailability for oral peptide candidates has been reported from clinical trials when either salcaprozate sodium (SNAC) or sodium caprate (C-10) is incorporated in the formulation. However, little is known about how they physically interact with peptides in solution. Our objective was to compare the biophysical interactions between the GLP-1 analogue exenatide (Byetta (R), Lilly), and C-10 or SNAC using a variety of advanced analytical techniques. First, critical micelle con-centration was measured in different buffers for both PEs. Dynamic light scattering (DLS) measurements revealed specific supramolecular structures arising from exenatide-PE association. Surface plasmon resonance (SPR) indicated the formation of exenatide-PE complexes with a high contribution from non-specific interactions and rapid binding kinetics, resulting in overall low affinities. DLS and isothermal titration calorimetry (ITC) were used to examine the supramolecular organization of the PEs, and revealed thermodynamic signatures charac-terized by unfavourable enthalpic contributions compensated by favourable entropic ones, but with low-affinity estimates in water (K-D in the 10-100 mu M range). With affinity capillary electrophoresis (ACE), weak interactions between exenatide and SNAC or C-10 were confirmed in saline, with a dissociation constant around 10 mu M and 30 mu M respectively. In biorelevant intestinal media, the bile salts in FaSSIF and FeSSIF further reduced the binding of both agents to exenatide (K-D asymptotic to 100 mu M), indicating that the interaction between the PEs and exenatide might be inhibited by bile salts in the GI lumen. This study suggests that the interactions of both PEs with exenatide follow a similar non-covalent mechanism and are of low affinity [3].
Intestinal Permeation Enhancers
Salcaprozate sodium (SNAC) and sodium caprate (C-10) are two of the most advanced intestinal permeation enhancers (PEs) that have been tested in clinical trials for oral delivery of macromolecules. Their effects on intestinal epithelia were studied for over 30 years, yet there is still debate over their mechanisms of action. C-10 acts via openings of epithelial tight junctions and/or membrane perturbation, while for decades SNAC was thought to increase passive transcellular permeation across small intestinal epithelia based on increased lipophilicity arising from non-c
- Related articles
- Related Qustion
- Salcaprozate Sodium: Revolutionizing Oral Drug Delivery through Enhanced Gastrointestinal Absorption May 15, 2024
Salcaprozate sodium is a groundbreaking compound in the field of drug delivery, offering new possibilities for the oral administration of peptides.
- Salcaprozate Sodium: Uses, Side Effects and Related Research Mar 15, 2024
Salcaprozate Sodium (SNAC) is a synthetic N-acetylated amino acid derivative of salicylic acid widely used as a carrier for orally administered drugs.
- Salcaprozate Sodium Facilitates Oral Delivery of Low-Permeability Drugs Jan 3, 2024
Salcaprozate sodium enhances API absorption, potentially improving drug efficacy and bioavailability. Extensively studied in oral formulations, it is deemed safe by the FDA.
2,4,6-Trichloroaniline is used as a raw material for azo dyes and basic fuchsin coupling agents for photography.....
Nov 14,2022Chemical pesticides ?Clenbuterol hydrochloride, the appearance of white crystalline powder, drug names such as dichlorolamine, sulventamine, claributerol, etc.....
Nov 15,2022DrugsSalcaprozate Sodium
203787-91-1You may like
Salcaprozate Sodium manufacturers
- sodium,8-[(2-hydroxybenzoyl)amino]octanoate
-
![203787-91-1 sodium,8-[(2-hydroxybenzoyl)amino]octanoate](/ProductImageEN/2024-05/Small/92dd275b-55a3-4a0f-ba23-248cbf8ca83e.jpg)
- $5.00 / 1KG
- 2024-05-11
- CAS:203787-91-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
- SNAC
-
- $0.00 / 1kg
- 2024-05-11
- CAS:203787-91-1
- Min. Order: 0.10000000149011612kg
- Purity: ≥99%
- Supply Ability: 20tons
- Salcaprozate sodium
-

- $9.00 / 10g
- 2024-05-11
- CAS:203787-91-1
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 10 tons