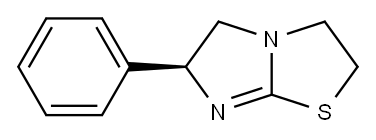
Synthesis and Toxicology of Levamisole
Oct 11,2022
General description
Levamisole is also known as levamisole, levothiamidazole hook roundworm, deworming speed. It is the levorotatory isomer of thiamidazole, and its hydrochloride or phosphate is commonly used. It is a white or off-white crystalline powder; odorless, bitter in taste, easily soluble in water, easily soluble in ethanol, and phosphate Slightly soluble. It is easy to decompose and fail in alkaline aqueous solution.

Synthetic routes
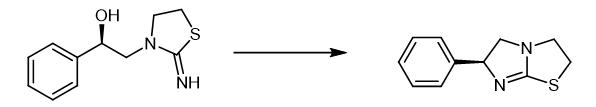
Fig. 2 The synthetic method 1 of Levamisole.
Get (S)-3-(2-hydroxy-2-phenylethyl)-2-iminothiazolidinone hydrochloride 25.850 g and join in the four-necked flask with mechanical stirring, add 25.850 g content and be 36.50% Hydrochloric acid, water bath control temperature 45℃-50℃, slowly add 17.50 g of chlorosulfonic acid dropwise with stirring, strictly control the reaction temperature at 45℃-50℃ during the dropwise addition, after adding, cool down to 10℃ with ice water, then The pH was slowly adjusted to 9.5-10.5 with an aqueous NaOH solution with a mass percentage concentration of 35%, and after the pH value was stable, filtration was performed to obtain 20.40 g of tetraimidazole free base with a yield of 98.50% and a purity of 98.62% [1].
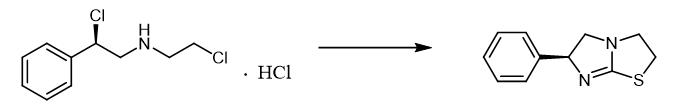
Fig. 3 The synthetic method 2 of Levamisole.
In a 50 mL reaction flask, 2.0 g compound ((R)-2-chloro-N-(2-chloroethyl)-2-phenylethan-1-amine), 30 g ethanol, 0.7 g thiourea and 1.0g sodium hydroxide were added, and the reaction was heated to 80-90℃ for 5 h. The solvent was removed under reduced pressure, and the yellow solid-liquid mixture was obtained. By silica gel column chromatography, 1.4 g yellow solid compound was obtained with a yield of 88% [2].
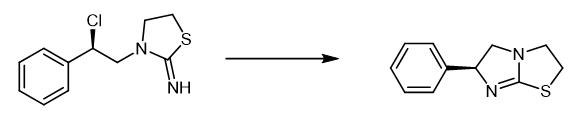
Fig. 4 The synthetic method 3 of Levamisole.
In a 250 mL reaction flask, 18.0 g compound ((R)-3-(2-chloro-2-phenylethyl)thiazolidin-2-imine), 80 g water, 80 g ethanol and 3.1 g sodium hydroxide were added, then heated to the reflux reaction for 2 h. Ethanol was removed under reduced pressure, then cooled to 20-30℃ and filtered to obtain a light yellow solid 12.2 g with a yield of 92% [2].

Fig. 5 The synthetic method 4 of Levamisole.
Add (-)-tetramisole HCl (200 mg, 0.83 mmol) to a 4-dram vial. Charge the vial with 2 mL diethyl ether. Treat the suspension with 4 M NaOH. Shake the suspension until the solid dissolves. Extract the aqueous phase with diethyl ether (4 × 3 mL). Dry the ethereal layer over anhydrous Na2SO4. Filter the ethereal layer. Evaporate the ethereal layer to dryness [3].
Toxicology
Levamisole has been identified as a cocaine adulterant in the United States since 2002. Although there is a variation in the percentage of levamisole in cocaine samples between European countries, measurement of levamisole in human samples of cocaine users has become increasingly important. To our best knowledge, only five deaths are reported (one twice) as a result of complications secondary to levamisole-tainted cocaine and none of these cases reports the post-mortem levamisole concentration. In this article, we present the post-mortem levamisole concentrations in fluids and tissues in two young cocaine users, dead after levamisole-adulterated cocaine intake. With the dearth of levamisole reported concentrations in literature, this particular report is of interest to the forensic toxicological and pathological communities. This article aims to be a supplementary alert to aware the risk that may occur using levamisole-adulterated cocaine and an incentive to publication of toxicity reports and new researches involving the combination of levamisole and cocaine [4].
Levamisole is an anthelmintic agent and also immunostimulant drug which is used to treat colorectal cancer. The present study aimed to show accidental consumption of levamisole alone induced multifocal inflammatory leukoencephalopathy. A 53-year-old male was admitted to the Neurology Department of Farabi Hospital (Kermanshah, Iran) with walking inability and recognition disorder. Following clinical examinations, the patient diagnosed as multifocal inflammatory leukoencephalopathy following levamisole consumption. The patient was treated with intravenous methylprednisolone followed by prednisolone. The magnetic resonance imaging (MRI) was done 1 month later and did not show a reduction or remission in the lesions. History of the patient showed that he had accidentally consumed levamisole 8 months ago. It seems that the consumption of levamisole can induce multifocal inflammatory leukoencephalopathy and delayed treatment of the patient with corticosteroid cannot diminish the neurotoxicity of levamisole. In addition, the cytotoxic dose of levamisole induces irreversible multifocal inflammatory leukoencephalopathy [5].
Pharmacokinetics
To investigate the pharmacokinetics of levamisole and a metabolite, p-hydroxylevamisole in patients with colorectal cancer treated with 5-fluorouracil (5-FU). Methods: Following an intravenous bolus dose of 5-FU, 20 patients with colorectal cancer received oral doses of 50 mg levamisole every 8 h for 3 days. Immediately after the last dose, blood and urine samples were collected over at least an 8-h period. Samples were assayed for levamisole and p-hydroxylevamisole by GC/MS. The levamisole plasma and urine data were subjected to pharmacokinetic analysis using NONMEM software. Results: Substantial interpatient variability was observed in the levamisole plasma concentration-time curves. Patients with cardiovascular or gastrointestinal complications demonstrated altered absorption of levamisole. Pharmacokinetic parameter values for levamisole were similar to those obtained previously in healthy subjects and other cancer patients. Conclusions: There is no evidence that the pharmacokinetics of levamisole are altered by 5-FU administered immediately prior to levamisole administration. The relationship between the substantial intersubject variability in levamisole plasma concentration-time curves and clinical outcome following 5-FU/levamisole adjuvant chemotherapy should be examined [6].
Clinical Trials
Levamisole exposure in cocaine users is a well-recognized cause of retiform purpura, a distinctive net-like maculopapular patch. Prolonged exposure to levamisole can lead to a serious systemic syndrome known as levamisole-induced vasculitis, most commonly involving the kidneys and lungs. More recently, retiform purpura has been observed in patients with the novel coronavirus disease of 2019 (COVID-19). Due to their overlapping dermatologic and systemic manifestations, levamisole-induced and COVID-19-induced retiform purpura may mimic one another in clinical presentation. The possibility that patients may present with one or both syndromes creates a diagnostic challenge. This review of levamisole-induced and COVID-19-induced retiform purpura highlights their corresponding and distinctive features. Additionally, we propose a unique staging system for levamisole-induced retiform purpura that may be valid for future classification of COVID-19-induced retiform purpura [7].
The efficacy of a drench containing praziquantel in combination with levamisole was evaluated in four trials performed in the 1990-91 and 1992-93 seasons in the Waikato Region of New Zealand. The trials involved 93 naturally infected lambs and compared the efficacy of a 3.75 mg/kg praziquantel - 7.5mg/kg levamisole drench against Moniezia expansa, with albendazole, an albendazole-levamisole combination and oxyclozanide-levamisole combination in controlled trials. There was no significant reduction in the number of Moniezia expansa scoleces or proglottids in the control, albendazole and albendazole-levamisole groups. Oxyclozanide gave a high clearance of proglottids, but a 28% reduction of scoleces. The praziquantel-levamisole combination demonstrated complete removal of segments in all trials, and of scoleces in two trials. One scolex was found in each of the two other trials. The combination was also tested for efficacy against nematode parasites. Total worm counts indicated that levamisole in the praziquantel-levamisole combination drench retained its efficacy. The economic benefits of the use of the praziquantel-levamisole drench were investigated. This trial compared the liveweight gains of three groups of 100 lambs treated twice 4 weeks apart with either levamisole or with the praziquantel-levamisole combination or acting as untreated control group. The group treated with the praziquantel-levamisole combination gained significantly more weight in both 4-week periods and overall, when compared with either the control or levamisole treated animals [8].
References
[1] Wang Y, Li Q, Qiao J, et al. Method for synthesizing tetramisole hydrochloride[P]. Faming Zhuanli Shenqing, 111138457, 2020.
[2] Li Z, Li Y, Li Zhen, et al. Green preparation of levamisole hydrochloride[P]. Faming Zhuanli Shenqing, 114315866, 2022.
[3] Sheppard C I, Taylor J L, Wiskur S L. Silylation-based kinetic resolution of monofunctional secondary alcohols[J]. Organic Letters, 2011, 13(15): 3794-3797.
[4] Indorato F, Romano G, Barbera N. Levamisole-adulterated cocaine: Two fatal case reports and evaluation of possible cocaine toxicity potentiation[J]. Forensic science international, 2016, 265: 103-106.
[5] Sariaslani P, Ghanbari A, Ghanbari P. Multifocal inflammatory leukoencephalopathy induced by accidental consumption of levamisole: A case report[J]. Iranian Journal of Neurology, 2012, 11(2): 65.
[6] Gwilt P, Tempero M, Kremer A, et al. Pharmacokinetics of levamisole in cancer patients treated with 5-fluorouracil[J]. Cancer chemotherapy and pharmacology, 2000, 45(3): 247-251.
[7] Keim C K, Schwartz R A, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes[J]. Archives of Dermatological Research, 2021: 1-9.
[8] Southworth J, Harvey C, Larson S. Use of praziquantel for the control of Moniezia expansa in lambs[J]. New Zealand veterinary journal, 1996, 44(3): 112-115.
- Related articles
- Related Qustion
Levamisole
14769-73-4You may like
- levamisole
-

- $10.00 / 1ASSAYS
- 2024-05-18
- CAS:14769-73-4
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton
- Levamisole
-

- $0.00 / 1G
- 2024-05-17
- CAS:14769-73-4
- Min. Order: 2G
- Purity: 99%
- Supply Ability: 20
- Levamisole
-

- $10.00 / 1kg
- 2024-05-17
- CAS:14769-73-4
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1200tons