The review of Cesium carbonate
Jan 28,2022
Introduction
Many of the properties of cesium carbonate are due to the softness of the cesium cation. This softness makes cesium carbonate rather soluble in organic solvents such as alcohols, DMF and Et2O. This has rendered cesium carbonate useful in palladium chemistry, which is often carried out in non-aqueous media where insolubility of inorganic bases can limit reactivity. Cs2CO3 has, for example, been used with good results in Heck, Suzuki and Sonogashira reactions.
Cesium carbonate has also received much attention for its use in O-alkylations, particularly of phenols. It has been postulated that O-alkylations of phenols using Cs2CO3 in non-aqueous solvents occurs via the ‘naked’ phenolate anion, which behaves as a strong nucleophile. Therefore, this methodology can even be applied to secondary halides, minimizing the usual unwanted side reactions such as elimination and decomposition.
Cesium carbonate has also found much use in solid supported synthesis, where solubility can be of importance. It has been reported that it not only promotes successful carbonylation of alcohols and carbamination of amines, but also suppresses common side reactions traditionally encountered with other protocols.
In peptide chemistry, a very mild way to produce esters of amino-protected peptides is to treat the carboxylic acid with cesium carbonate followed by the addition of a halide in DMF. An intramolecular version has been used to produce macrocyclic lactones.
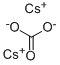
Introduction Cesium carbonate is a white hygroscopic powder that is readily soluble in water. It is produced by reacting cesium hydroxide with carbon dioxide1 (Picture 1).
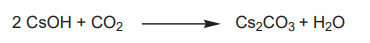
Picture 1 Preparation of cesium carbonate.
The application of cesium carbonate
Many of the properties of cesium carbonate are due to the softness of the cesium cation. This softness makes cesium carbonate rather soluble in organic solvents such as alcohols, DMF and Et2O[1]. This has rendered cesium carbonate useful in palladium chemistry, which is often carried out in non-aqueous media where insolubility of inorganic bases can limit reactivity. Cs2CO3 has, for example, been used with good results in Heck, Suzuki and Sonogashira reactions[2-5]. Cesium carbonate has also received much attention for its use in O-alkylations, particularly of phenols.It has been postulated that O-alkylations of phenols using Cs2CO3 in non-aqueous solvents occurs via the ‘naked’ phenolate anion, which behaves as a strong nucleophile. Therefore, this methodology can even be applied to secondary halides, minimizing the usual unwanted side reactions such as elimination and decomposition[6-7].
Different solid supported synthesis using cesium carbonate
Cesium carbonate has also found much use in solid supported synthesis, where solubility can be of importance. It has been reported that it not only promotes successful carbonylation of alcohols and carbamination of amines, but also suppresses common side reactions traditionally encountered with other protocols[8]. In peptide chemistry, a very mild way to produce esters of amino-protected peptides is to treat the carboxylic acid with cesium carbonate followed by the addition of a halide in DMF[9]. An intramolecular version has been used to produce macrocyclic lactones[10].
(A) Fu and co-workers have used Cs2CO3 as the base in Suzuki cross-coupling reactions (picture 2) with yields up to 86%. When the same reactions were performed with Na2CO3 or NEt3, the yields were 29% and 50%, respectively [4].
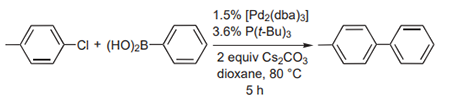
Picture 2 cross-coupling reactions
(B) Littke and Fu have also shown the superiority of Cs2CO3 as compared with other bases in the Heck coupling of methylacrylate with chlorobenzene. K2CO3, NaOAc, NEt3, K3PO4 and Cs2CO3 were used to provide yields of only 9%, 21%, 37%, 50% and 56%, respectively (picture 3 )[1-2].
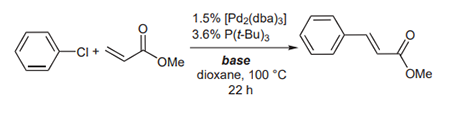
Picture 3 The superiority of Cs2CO3
(C) In the alkylation of phenols, Parrish and coworkers have shown the utility of Cs2CO3. Its use makes the alkylation possible even with highly reactive halides which, under other conditions, are prone to eliminations or other side reactions (picture 4) [6].
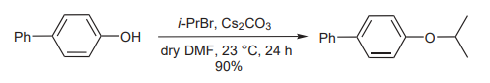
Picture 4 The utility of Cs2CO3
(D) In natural product chemistry, Fujivara et al. have used Cs2CO3 in the key ring-forming step in the synthesis of lipogrammistin-A, originally isolated from the skin mucus of the grammistid fish (picture 5)[11].
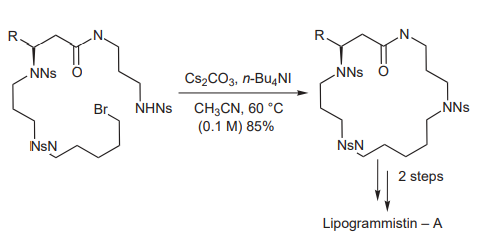
Picture 5 The synthesis of lipogrammistin-A
(E) Salvatore et al. used Cs2CO3 when constructing carbonates (not shown) and carbamates in good yield on solid support under CO2 atmosphere and with TBAI as a co-catalyst (picture 6)[8].
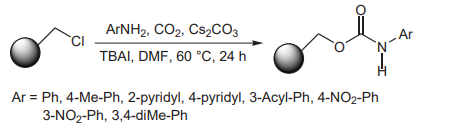
Picture 6 Constructing carbonates and carbamates
(F) Walla and Kappe have shown the utility of Cs2CO3 as a base when connecting benzoic acids to Merrifield resins under microwave irradiation (picture 7)[12].
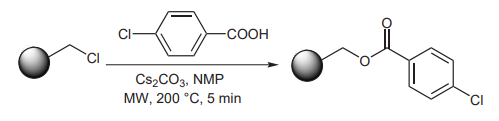
Picture 7 The utility of Cs2CO3 as a base
(G) Large macrocycles can be prepared using Cs2CO3. The reagent serves both as a base and as a cation template in the macrocyclization of dicarboxylic acids and dihalides to generate the desired crown ethers[10].
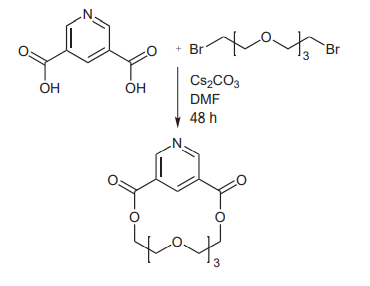
Picture 8 Large macrocycles can be prepared using Cs2CO3
Reference
- Related articles
- Related Qustion
- Cesium Carbonate Promoted Direct Amidation of Unactivated Esters with Amino Alcohol Derivatives Apr 17, 2024
Cesium carbonate is a white hygroscopic powder that is readily soluble in water.
- Application and Property of Cesium carbonate Sep 8, 2023
Cesium carbonate is a basic carbonate used in organic synthesis as a mild inorganic base.
- Synthesis of Cesium carbonate Aug 11, 2021
Cesium carbonate can be used as a modified layer between the active layer of the solar cell and the Al electrode.
Lithium aluminium hydride (LiAl H4), commonly abbreviated to LAH, is a powerful reducing agent used in organic chemistry.....
Jan 27,2022Catalyst and AuxiliaryHydroxytyrosol and its derivatives possess antioxidant, antimicrobial, and anti-inflammatory activities and beneficial effects on the cardiovascular system by preventing oxidative stress situations.....
Jan 28,2022APICesium carbonate
534-17-8You may like
Cesium carbonate manufacturers
- Cesium carbonate
-

- $30.00 / 1kg
- 2024-05-27
- CAS:534-17-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Caesium carbonate
-

- $9.90/ kg
- 2024-05-27
- CAS:534-17-8
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 1000000000 ton
- Cesium carbonate
-

- $0.00 / 25kg
- 2024-05-16
- CAS:534-17-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month