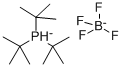Tri-tert-butylphosphine tetrafluoroborate: Applications in Synthesis of Multisubstituted Indoles and its Synthesis Method
May 11,2024
General Description
Tri-tert-butylphosphine tetrafluoroborate, with a molecular formula of C12H28PBF4 and a molecular weight of 290.13, is a vital phosphorus ligand catalyst used in asymmetric conjugate addition reactions. Its applications include facilitating the copper(ii)-catalyzed synthesis of multisubstituted indoles, leading to the production of complex indole structures. Tri-tert-butylphosphine tetrafluoroborate enhances the efficiency of copper catalysts in N-arylation and cross-dehydrogenative coupling reactions, resulting in the formation of indole-3-carboxylic esters. The synthesis method involves a series of steps to achieve a high yield product, avoiding the isolation of sensitive intermediates. Safety precautions must be taken when handling this chemical to prevent health hazards.

Figure 1. Tri-tert-butylphosphine tetrafluoroborate
Overview
Tri-tert-butylphosphine tetrafluoroborate, a highly specialized organic phosphorus compound, boasts a unique molecular formula of C12H28PBF4 and a molecular weight of 290.13. Its melting point stands at 261°C, indicating its thermal stability. This compound finds application as a highly active phosphorus ligand catalyst, especially in asymmetric conjugate addition reactions involving carbonyl compounds. Its dual-phosphine chiral ligands induce reactions along highly enantioselective pathways, enabling the controlled synthesis of chiral compounds. While handling this chemical, it is essential to maintain safety measures such as wearing protective gear and ensuring adequate ventilation to prevent any potential health hazards. 1
Applications in Synthesis of Multisubstituted Indoles
Tri-tert-butylphosphine tetrafluoroborate plays a crucial role in the copper(ii)-catalyzed synthesis of multisubstituted indoles via sequential Chan-Lam and cross-dehydrogenative coupling reactions. Initially, in the Chan-Lam N-arylation step, arylboronic acids and ester (Z)-3-aminoacrylates react to form ester (Z)-3-(arylamino)acrylate intermediates. This reaction takes place in DMF at 100 °C for 24 hours, facilitated by Cu(OAc)2/tri-tert-butylphosphine tetrafluoroborate, with myristic acid as an additive, and KMnO4 and KHCO3 as additional components. Subsequently, these intermediates undergo an intramolecular oxidative cross-dehydrogenative coupling process. This step occurs in mixed solvents (DMF/DMSO = 2 : 1) at 130 °C. Tri-tert-butylphosphine tetrafluoroborate enhances the efficiency of the copper catalyst in both the initial N-arylation and the subsequent cross-dehydrogenative coupling reactions. The resulting product is a diverse range of indole-3-carboxylic esters, characterized by C3-functionalized multi-substituted indole derivatives. Tri-tert-butylphosphine tetrafluoroborate not only acts as a ligand for copper but also contributes to the overall yield and selectivity of the desired indole products. This methodology provides a versatile approach for the synthesis of complex indole structures, showcasing the significance of Tri-tert-butylphosphine tetrafluoroborate in modern organic synthesis. 2
Synthesis Method
The synthesis method for tri-tert-butylphosphine tetrafluoroborate involves several steps to achieve a high yield of the product. Here's a detailed description of the process: Firstly, in a dried 500-mL three-neck flask equipped with necessary apparatus, CuBr-Me2S and LiBr are added. The reaction vessel is purged with N2, followed by the addition of hexane. Phosphorus trichloride is then added to the suspension, and the reaction is cooled with an ice bath. Next, tert-Butylmagnesium chloride (t-BuMgCl) in diethyl ether (Et2O) is added dropwise, while maintaining the internal temperature below 8 °C during the initial 50 mL addition. The flask is then warmed with a water bath, and the remainder of the t-BuMgCl solution is added. The mixture is stirred vigorously for 13 hours at 23 °C, then recooled with an ice bath. Aqueous HBF4 solution is added carefully, keeping the internal temperature below 25 °C. After stirring the biphasic mixture and filtering over Celite, the layers are separated. The aqueous layer is washed with hexane to remove impurities. Subsequently, the aqueous layer is extracted with CH2Cl2, and the combined CH2Cl2 layers are dried with MgSO4, filtered, and evaporated in vacuo. The product is then crystallized using ethanol, with a concentration and further crystallization of the mother liquor. This procedure offers a convenient, simple, and high-yielding method for synthesizing tri-tert-butylphosphine tetrafluoroborate, avoiding the isolation of sensitive intermediates. 3
Reference
1. Tri-tert-butylphosphine tetrafluoroborate. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 44558591.
2. Chen X, Bian Y, Mo B, Sun P, Chen C, Peng J. Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan-Lam and cross-dehydrogenative coupling reactions. RSC Adv. 2020; 10(42): 24830-24839.
3. Saget, T, Cramer N. Convenient Preparation of Tri-tert-butylphosphonium Tetrafluoroborate. Synthesis-Stuttgart. 2011; 34(1): 2369-2371.
- Related articles
- Related Qustion
- Tri-tert-butylphosphine tetrafluoroborate: properties, applications and safety Sep 18, 2023
Tri-tert-butylphosphine tetrafluoroborate is a white crystalline solid used as a ligand in transition metal-catalyzed reactions, enhancing reactivity and selectivity.
- Convenient Preparation of Tri-tert-butylphosphine Tetrafluoroborate Nov 20, 2019
Tri-tert-butylphosphine tetrafluoroborate(CAS: 131274-22-1) also called Tri-tert-butylphosphonium tetrafluoroborate (TTBP ? HBF4) is used as a precursor to tri-tert-butylphosphine (TTBP).
2-Iodobenzoic acid enables synthesis of crucial compounds for medical advancement, enhancing diagnostic techniques and potential treatments in medicinal chemistry.....
May 11,2024API4-Pyridinecarboxaldehyde is a versatile building block in medicinal chemistry, offering high-yield synthesis and therapeutic potential in treating IPF and malaria.....
May 11,2024APITri-tert-butylphosphine tetrafluoroborate
131274-22-1You may like
Tri-tert-butylphosphine tetrafluoroborate manufacturers
- Tri-tert-butylphosphonium tetrafluoroborate
-
- $12.00 / 25g
- 2024-02-23
- CAS:131274-22-1
- Min. Order: 25g
- Purity: 0.98
- Supply Ability: 25kg
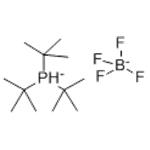
- Tri-tert-butylphosphine tetrafluoroborate
-
- $50.00 / 1KG
- 2023-12-23
- CAS:131274-22-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Tri-tert-butylphosphine tetrafluoroborate
-
- $0.00 / 1kg
- 2023-11-01
- CAS:131274-22-1
- Min. Order: 1kg
- Purity: 99.0%min
- Supply Ability: 800kg