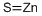Zinc Sulfide Nanoparticles: Unique Properties and Potential Applications in Biomedicine
Jan 22,2024
General Description
Zinc sulfide nanoparticles possess unique properties that make them attractive for various applications, including biomedical applications, optoelectronics, and energy storage. Their size-dependent autofluorescence and photocatalytic activity have been exploited for imaging, sensing, and cancer treatment. Zinc sulfide NPs are biocompatible, non-toxic, stable, and have a high surface area to volume ratio, making them useful as catalysts for chemical reactions. Additionally, their properties can be tuned by controlling their size, shape, and surface chemistry. Specifically, Zinc sulfide-NPs have shown promise in skin regeneration and wound healing, and as a theranostic modality for antitumorigenic therapy. Overall, Zinc sulfide nanoparticles have the potential to revolutionize various fields and transform the way we address critical problems in healthcare and technology.

Figure 1. Zinc sulfide
Unique Properties of Zinc Sulfide Nanoparticles
Zinc sulfide nanoparticles possess unique properties that make them attractive for a range of applications. Zinc sulfide NPs are semiconductor quantum dots with a size less than 10 nm, which confers them with size-dependent autofluorescence. When excited by energy higher than the bandgap, Zinc sulfide NPs exhibit photocatalysis and release reactive oxygen species (ROS), which can have cytotoxic consequences. Apart from their intrinsic photocatalytic properties, Zinc sulfide NPs are biocompatible, non-toxic, and stable. They have a high surface area to volume ratio, which makes them useful as catalysts for chemical reactions. Additionally, Zinc sulfide NPs are easy to synthesize, and their properties can be tuned by controlling their size, shape, and surface chemistry. In recent years, Zinc sulfide NPs have been investigated for their potential in various fields, including biomedical applications, optoelectronics, and energy storage. Their unique properties, such as fluorescence and photocatalysis, have been exploited for imaging, sensing, and cancer treatment. In summary, Zinc sulfide NPs possess a range of unique properties that make them attractive for various applications. These properties include size-dependent autofluorescence, photocatalytic activity, stability, biocompatibility, and tunability. 1
Biomedical Applications
Theranostic nanotool for cancer management
Zinc sulfide nanoparticles (NPs) have emerged as a promising material for various applications due to their unique properties. In a study, the researchers aimed to explore the potential of Zinc sulfide NPs as a theranostic modality for combating superficially accessible carcinoma. The size-dependent autofluorescence of Zinc sulfide NPs, which is a result of excitation of valence electrons by energy higher than the bandgap, was investigated. The researchers verified the photocatalytic activity of Zinc sulfide NPs and their ability to donate electrons upon UV excitation. They also tested the efficacy of UV-activated Zinc sulfide NPs in inducing reactive oxygen species (ROS)-dependent apoptosis in squamous cell carcinoma and breast cancer cell lines. The results showed that UV excitation of Zinc sulfide NPs generated energetic electron-hole pairs, leading to the release of ROS and subsequent apoptotic cancer cell deaths. This effect was found to be more potent compared to single treatment modalities of nonexcited nanoparticles and UV alone. Additionally, the luminescence of Zinc sulfide NPs allowed for visualization of their intracytoplasmic uptake and tracking of cellular response. In conclusion, the enhanced luminescence and cytotoxicity of photoactivated Zinc sulfide NPs make them promising candidates for theranostic applications, particularly in the field of antitumorigenic therapy. 2
Skin regeneration
Zinc sulfide nanoparticles have shown potential for use in wound healing applications. The current technologies used in wound healing often leave wounds with scarring and lacking skin accessories, highlighting the need for new clinical modalities. This study investigated the effects of Zinc sulfide-NPs on wound healing both in vitro and in vivo using rat models. The results showed that Zinc sulfide-NPs inhibited the proliferation of skin fibroblast cells and altered cytoskeletal organization, resulting in reduced collagen synthesis and contractile activity. Zinc sulfide-NPs also regulated redox homeostasis and promoted fibroblast viability in hypoxic conditions. In the rat full-thickness wound model, Zinc sulfide-NPs reduced wound contraction, enhanced re-epithelization, and promoted skin appendage formation. These findings suggest that Zinc sulfide-NPs have promising practical applications for topical or systemic treatment for wound repair. With their ability to regulate redox homeostasis and promote fibroblast viability, Zinc sulfide-NPs could potentially improve skin regeneration and reduce scarring in wound healing. Further research and development in this area could lead to significant advancements in wound care technology. 3
Reference
1. Wang G , Cen D , Ren Z , et al. Zinc sulfide nanoparticle-decorated fibre mesh to enable localized H2S-amplified chemotherapy. Chem Commun (Camb). 2020;56(31):4304-4307.
2. Essawy MM, Rafik ST, Awaad AK, Mourad GM, El Achy SN. Photo-excitable zinc sulfide nanoparticles: A theranostic nanotool for cancer management. Oral Dis. 2023;29(8):3243-3258.
3. Han B, Fang WH, Zhao S, Yang Z, Hoang BX. Zinc sulfide nanoparticles improve skin regeneration. Nanomedicine. 2020;29:102263.
- Related articles
- Related Qustion
- Zinc sulfide Crystal Nov 21, 2023
Zinc sulfide takes two types of structures: zinc blend and wurtzite structures. Cubic system and zinc-blend structure with a lattice constant of a=0.5412 nm and Zn–S=0.236 nm. It is called b-ZnS.
- What is Zinc sulfide used for? Dec 22, 2022
Zinc sulfide is an inorganic compound, the chemical formula is ZnS, the appearance is white or slightly yellow powder, and the color will become darker when exposed to light. It is stable in dry air, and it will gradually oxidize to zinc su
Bromelain, a protein found in pineapple stems, has diverse applications in medicine due to its antimicrobial properties, and potential in treating various conditions.....
Jan 22,2024APITert-butyl acetate is a readily available low-cost solvent produced industrially from acetic acid and isobutylene. Its good safety profile makes it suitable for widespread use in catalytic direct amidation reactions.....
Jan 22,2024Chemical ReagentsZinc sulfide
1314-98-3You may like
- Zinc sulfide
-

- $50.00/ kg
- 2024-04-18
- CAS:1314-98-3
- Min. Order: 1kg
- Purity: 99.912%
- Supply Ability: 10ton
- Zinc sulfide
-

- $6.00 / 1KG
- 2024-04-12
- CAS:1314-98-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000KG/Month
- Zinc sulfide
-
- $32.00 / 1kg
- 2024-03-29
- CAS:1314-98-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available