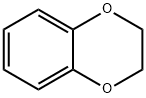
1,4-Benzodioxan synthesis
- Product Name:1,4-Benzodioxan
- CAS Number:493-09-4
- Molecular formula:C8H8O2
- Molecular Weight:136.15
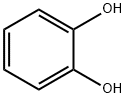
120-80-9
493 suppliers
$13.00/25g

107-06-2
400 suppliers
$14.00/25g

493-09-4
280 suppliers
$10.00/5 g
Yield:493-09-4 115 g
Reaction Conditions:
with sodium hydroxide in dimethyl sulfoxide at 80 - 85; for 5.45 h;
Steps:
2 Example 2: Preparation of ethylenedioxybenzene (Dimethyl enedioxybenzene) (compound of Formula IB)
Dimethylsulfoxide (DMSO, 360g) and 1,2-dichloroethane (280g) were charged into a 2 liter reaction flask. The mixture was heated to 80°C under stirring. 110 g of Catechol (in 5 g lots) and 148 g of sodium hydroxide (in 6.7 g lots) were simultaneously added in 22 lots each at intervals of 7 minutes maintaining the temperature of the reaction medium between 80°C and 85°C under stirring. After addition of catechol and alkali was complete, the reaction medium was stirred for 3 hrs till the catechol content was less than 0.1% by HPLC analysis. 200 ml water was charged into the reactor and ethyl enedioxbenzene and unreacted 1,2-dichloroethane were separated by azeotropic distillationusing a Dean-Stark apparatus.. The mixture of methylenedioxybenzene and 1,2-dichloroethane was distilled to give 115g (104.5% w/w on catechol) with purity >99.0% by GC analysis and recover 115 g of unreacted 1,2-dicloroethane. The moisture present in DMSO was removed and the DMSO was cooled to less than 30°C and filtered to remove salt and unreacted alkali. DMSO (315 g) was recovered from the mother liquor by distillation
References:
ANTHEA AROMATICS PRIVATE LIMITED;MOHAPATRA, Manoj Kumar;BENDAPUDI, Ramamohanrao;MENACHERRY, Paul Vincent;PAUL, Vincent WO2017/158404, 2017, A1 Location in patent:Page/Page column 14

34743-89-0
21 suppliers
$65.00/50mg

493-09-4
280 suppliers
$10.00/5 g

4792-78-3
68 suppliers
$60.00/50 g

493-09-4
280 suppliers
$10.00/5 g

120-80-9
493 suppliers
$13.00/25g

106-93-4
375 suppliers
$15.00/5g

493-09-4
280 suppliers
$10.00/5 g

3663-82-9
139 suppliers
$13.43/1gm:

493-09-4
280 suppliers
$10.00/5 g