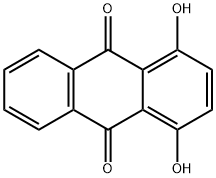
1,4-Dihydroxyanthraquinone synthesis
- Product Name:1,4-Dihydroxyanthraquinone
- CAS Number:81-64-1
- Molecular formula:C14H8O4
- Molecular Weight:240.21
Yield:81-64-1 96%
Reaction Conditions:
with boron trioxide;sulfuric acid at 125 - 160; for 16 h;Temperature;
Steps:
1; 2 Example 2:
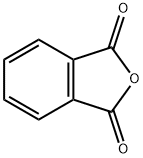
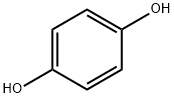
In a dry 500nl four-necked flask, 145 g (1.45 mol) of 98% sulfuric acid was added, 50.5 g (0.726 mol) of boric anhydride was added under stirring, and after the addition, stirred for 15 minutes to make it fully dissolved. Then 59.1 g of phthalic anhydride (molecular weight 148.1, 0.399 mol) and 40 g of hydroquinone (molecular weight 110.1, 0.363 mol) were added, and stirred for 15 minutes to fully dissolve. Begin to heat up with a heat-conducting oil furnace, heat up to 125°C, and keep it at 125-130°C for 8 hours. Then continue to heat up to 155°C, and keep it at 155-160°C for 8 hours. After the heat preservation, the temperature is lowered to 50-60°C, and the reaction materials are transferred to a four-neck flask filled with 730g of clear water. After the material transfer is completed, the temperature is controlled at 100-110°C, and the isolation is about 2 hours. Then the temperature is lowered to 60-80°C, filtered, washed with water, and dried to obtain the product. 85.20 g of 1,4-dihydroxyanthraquinone was obtained, the chromatographic content was 98.25%, and the yield was 96.0%.
References:
CN113072439,2021,A Location in patent:Paragraph 0020-0023
28736-42-7
94 suppliers
$36.00/250mg

81-64-1
447 suppliers
$5.00/25g
2289-36-3
2 suppliers
inquiry

81-64-1
447 suppliers
$5.00/25g