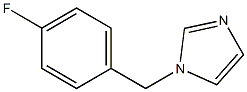
1-[(4-fluorophenyl)methyl]imidazole synthesis
- Product Name:1-[(4-fluorophenyl)methyl]imidazole
- CAS Number:56643-73-3
- Molecular formula:C10H9FN2
- Molecular Weight:176.1903
Yield:-
Reaction Conditions:
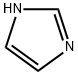
Stage #1: 1H-imidazolewith potassium carbonate in N,N-dimethyl-formamide; for 0.333333 h;
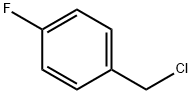
Stage #2: 1-chloromethyl-4-fluorobenzene in N,N-dimethyl-formamide at 50; for 2 h;
Steps:
4.1.6. 1-substituted benzyl-1H-imidazoles (7a-7d)
General procedure: A mixture of potassium carbonate (15.2 g, 0.11 mol), imidazole (6.8 g, 0.1 mol) in DMF (60 mL) was stirred for 20 min, then substituted benzyl chloride (0.11 mol) was added dropwise to the solution. The reaction mixture was stirred at 50 °C for 2 h and poured into water (250 mL). The aqueous layer was extracted with CH2Cl2 and the combined organic layer was washed with water repeatedly, and dried over Na2SO4. The solvent was removed under reduced pressure to afford title compounds 7a-7d as pale yellow oils.
References:
Jiang, Nan;Zhai, Xin;Zhao, Yanfang;Liu, Yajing;Qi, Baohui;Tao, Haiyan;Gong, Ping [European Journal of Medicinal Chemistry,2012,vol. 54,p. 534 - 541] Location in patent:experimental part