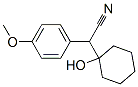
2-(1-HydroxyCyclohexyl)-2-(4-MethoxyPhenyl)Acetonitrile synthesis
- Product Name:2-(1-HydroxyCyclohexyl)-2-(4-MethoxyPhenyl)Acetonitrile
- CAS Number:93413-76-4
- Molecular formula:C15H19NO2
- Molecular Weight:245.32

108-94-1
524 suppliers
$12.00/50g

93413-76-4
241 suppliers
$22.00/100mg
Yield:93413-76-4 90%
Reaction Conditions:
Stage #1:p-methoxybenzylnitrile with sodium hydroxide;polyethylene glycol in water at 0 - 5; for 2 h;
Stage #2:cyclohexanone with sodium hydroxide in water at 0 - 20; for 13.5 h;
Steps:
A
Example (A):Preparation of l-cyano-[(4-methoxyphenyl)methyl]cyclohexanol (II): 4-Methoxyphenyl acetonitrile (100 gm) was cooled to 0-5 0C and to this was added 10% aqueous solution of sodium hydroxide (13.6 gm) followed by PEG-400 (7.3 ml). The reaction mixture was further stirred for 2 hours at 0-5 0C. Then first lot of cyclohexanone (49.1 ml) was added and reaction mixture was further stirred for 1-1.5 hours. Then the second lot of sodium hydroxide (13.6 gm) and cyclohexanone (49.1 ml) was added to the reaction mixture. The^n the temperature of the reaction mixture was allowed to attain the ambient temperature and was stirred for 10-12 hours. The reaction progress was monitored by TLC. After the completion of the reaction (TLC), hexane (500 ml) was added to the reaction mixture and stirred for further 15 min. The reaction mixture was filtered and the collected solid was washed with n-hexane (200 ml) and water (200 ml). The resulting solid was dried at 55-60 0C. The dried product was l-cyano-[(4- methoxyphenyl)methyl]cyclohexanol (II) (150 gm, 90%) in >99.5% purity. 1H (CDCl3): 7.3 and 6.9 (q, 4H), 3.8 (s, 3H), 3.75 (s, IH)5 1.55 (m, 10H)
References:
UNICHEM LABORATORIES LIMITED WO2007/94008, 2007, A2 Location in patent:Page/Page column 7

108-94-1
524 suppliers
$12.00/50g
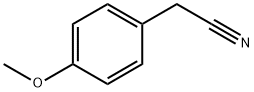
104-47-2
456 suppliers
$6.00/10g

93413-76-4
241 suppliers
$22.00/100mg

108-94-1
524 suppliers
$12.00/50g
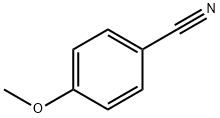
874-90-8
340 suppliers
$8.00/10g

93413-76-4
241 suppliers
$22.00/100mg

104-47-2
456 suppliers
$6.00/10g

93413-76-4
241 suppliers
$22.00/100mg

874-90-8
340 suppliers
$8.00/10g

93413-76-4
241 suppliers
$22.00/100mg