1-m-tolyl-1H-1,2,4-triazole-3-carboxylic acid synthesis
- Product Name:1-m-tolyl-1H-1,2,4-triazole-3-carboxylic acid
- CAS Number:1245649-64-2
- Molecular formula:C10H9N3O2
- Molecular Weight:203.2
Yield:1245649-64-2 27%
Reaction Conditions:
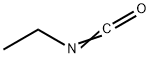
Stage #1: 1-amino-3-methylbenzenewith hydrogenchloride;sodium nitrite in water at 0; for 0.25 h;
Stage #2: ethyl isocyanatewith sodium acetate in ethanol;water; for 2 h;
Steps:
Intermediate 2: 1-m-Tolyl-1H-[1,2,4]-triazole-3-carboxylic acid Method A
Intermediate 2: 1-m-Tolyl-1H-[1,2,4]-triazole-3-carboxylic acid
Method A
(According to K. Matsumoto et al., Synthesis 1975, 609-610.):
A solution of 1.61 g (23.3 mmol) sodium nitrite in water was added dropwise to a solution of 2.50 g (23.3 mmol) m-toluidine in 4 M hydrochloric acid at 0° C.
The resulting mixture was stirred at 0° C. for 15 min, before a solution of 9.57 g (117 mmol) sodium acetate in 10 mL water was added, followed by addition of 2.55 mL (23.3 mmol) ethyl isocyanate and 50 mL EtOH.
The resulting mixture was stirred for 2 h.
The solvent was removed by distillation and the residue taken up in MeOH/water.
1 M lithium hydroxide solution was added and the mixture was stirred at RT.
When saponification was complete, MeOH was removed by distillation and the product was precipitated with conc. HCl, filtered and washed with water.
The crude product was purified by trituration with diethyl ether.
yield: 1.44 g (27%)
ESI-MS: m/z=204 (M+H)+
References:
US2013/158042,2013,A1 Location in patent:Paragraph 0223; 0224; 0225; 0226; 0227
2999-46-4
219 suppliers
$13.43/1gm:
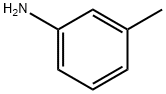
108-44-1
366 suppliers
$9.00/1g

1245649-64-2
11 suppliers
inquiry