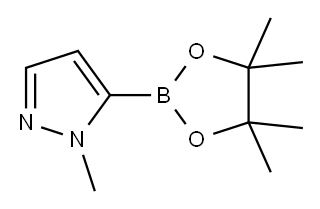
1-Methyl-1H-pyrazole-5-boronic acid pinacol ester synthesis
- Product Name:1-Methyl-1H-pyrazole-5-boronic acid pinacol ester
- CAS Number:847818-74-0
- Molecular formula:C10H17BN2O2
- Molecular Weight:208.07
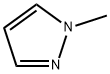
930-36-9
324 suppliers
$5.00/1g
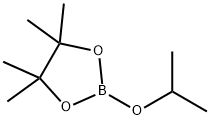
61676-62-8
311 suppliers
$10.00/5g

847818-74-0
263 suppliers
$6.00/1g
Yield:847818-74-0 77%
Reaction Conditions:
Stage #1:1-methyl-1H-pyrazole with n-butyllithium in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at -78 - 0; for 1.25 h;
Stage #3: with ammonium chloride in tetrahydrofuran
Steps:
2
To a solution of 1-methyl pyrazole (4.1 g, 50 mmole) in THF (100 mL) at 00C was added n-BuLi (2.2M in THF, 55 mmole). The reaction solution was stirred for 1 hour at RT and then cooled to -78°C [J. Heterocyclic Chem. 41 , 931 (2004)]. To the reaction solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (12.3 mL, 60 mmole). After 15 min at -78°C, the reaction was allowed to warm to 00C over 1 hour. The reaction
References:
SMITHKLINE BEECHAM CORPORATION WO2009/158372, 2009, A1 Location in patent:Page/Page column 30; 31
5419-55-6
369 suppliers
$12.00/5g

847818-74-0
263 suppliers
$6.00/1g
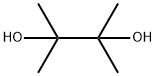
76-09-5
373 suppliers
$8.19/250mg

720702-41-0
174 suppliers
$12.00/250mg

847818-74-0
263 suppliers
$6.00/1g

930-36-9
324 suppliers
$5.00/1g
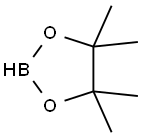
25015-63-8
216 suppliers
$22.39/5G

847818-74-0
263 suppliers
$6.00/1g

930-36-9
324 suppliers
$5.00/1g

25015-63-8
216 suppliers
$22.39/5G

847818-74-0
263 suppliers
$6.00/1g
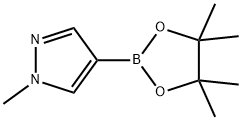
761446-44-0
351 suppliers
$10.00/1g