
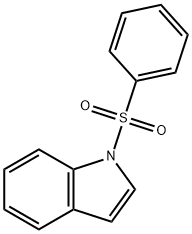
1-(PHENYLSULFONYL)PYRROLE synthesis
- Product Name:1-(PHENYLSULFONYL)PYRROLE
- CAS Number:16851-82-4
- Molecular formula:C10H9NO2S
- Molecular Weight:207.25
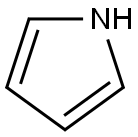
109-97-7
333 suppliers
$9.00/5 g
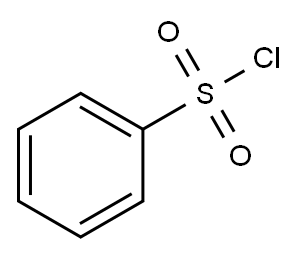
98-09-9
340 suppliers
$12.00/25 g

16851-82-4
163 suppliers
$11.00/1g
Yield:16851-82-4 99%
Reaction Conditions:
with tetra(n-butyl)ammonium hydrogensulfate;sodium hydroxide in dichloromethane;water at 20;Inert atmosphere;Cooling;
Steps:
1-(Phenylsulfonyl)-1H-pyrrole 14
To a cooled solution of aqueous sodium hydroxide (50% w/v, 20 mL, 250 mmol) and dichloromethane (20 mL) was added tetrabutylammonium hydrogen sulfate (250 mg, 750 μmol) and freshly distilled pyrrole (1.0 g, 14.9 mmol) followed by benzenesulfonyl chloride (2.1 mL, 16.4 mmol). The mixture was stirred at room temperature for 3 hours, quenched with saturated aqueous ammonium chloride (200 mL) and extracted with dichloromethane (3 x 40 mL). The combined organic extracts were washed with saturated aqueous sodium hydrogen carbonate (2 x 50 mL) and saturated aqueous sodium chloride (50 mL), dried over magnesium sulfate and concentrated to afford the desired product (3.1 g, 99%) as an off-white solid, mp: 88.5-89.3 °C; Rf: 0.47 (1:4 diethyl ether, hexane); IR (vmax (film)): 2962, 2875, 1482, 1450, 1365, 1314, 1168, 726 cm-1; 1H NMR (300 MHz, CDCl3): δ 6.30 (2H, t, J = 2.1 Hz), 7.17 (2H, t, J = 2.1 Hz), 7.44-7.55 (2H, m), 7.55-7.70 (1H, m), 7.81-7.89 (2H, m) ppm; 13C NMR (75 MHz, CDCl3): δ 113.9, 121.1, 127.0, 129.6, 134.1, 139.4 ppm.
References:
Moir, Michael;Boyd, Rochelle;Gunosewoyo, Hendra;Montgomery, Andrew P.;Connor, Mark;Kassiou, Michael [Tetrahedron Letters,2019,vol. 60,# 36,art. no. 151019] Location in patent:supporting information
696-59-3
374 suppliers
$7.00/25g
98-10-2
332 suppliers
$21.00/25g

16851-82-4
163 suppliers
$11.00/1g

25630-24-4
12 suppliers
$158.00/1g

16851-82-4
163 suppliers
$11.00/1g

16851-71-1
7 suppliers
inquiry

16851-71-1
7 suppliers
inquiry

16851-82-4
163 suppliers
$11.00/1g

696-59-3
374 suppliers
$7.00/25g

98-10-2
332 suppliers
$21.00/25g
40899-71-6
148 suppliers
$5.00/1g

16851-82-4
163 suppliers
$11.00/1g