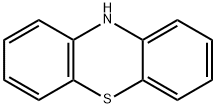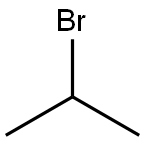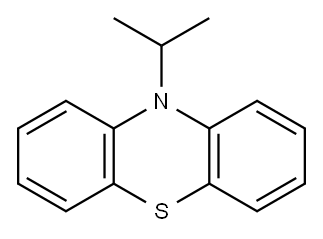
10-(propan-2-yl)-10H-phenothiazine synthesis
- Product Name:10-(propan-2-yl)-10H-phenothiazine
- CAS Number:17427-04-2
- Molecular formula:C15H15NS
- Molecular Weight:241.3513
Yield:17427-04-2 82.6 %
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
4
Under an inert gas atmosphere, phenothiazine HPT (0.1g, 5.0mmol, 1.0eq.) and sodium hydride (0.3g, 11.0mmol, 2.2eq.) were dissolved in anhydrous DMF solution at 0°C and stirred for 10min , Add bromoisopropane (1.4mL, 15.0mmol, 3.0eq.) to the reaction mixture, stir at room temperature for 16h after the reaction is complete, add ice water to the reaction solution, extract the reaction solution with dichloromethane, collect organic phase, then dried with anhydrous Na2SO4, and spin-dried to obtain a crude product.Using petroleum ether as eluent, the crude product was separated by silica gel column chromatography and recrystallized from methanol to obtain 10-isopropyl-10H-phenothiazine HH-IPT (1.0 g, 82.6%) as a colorless solid.Then 10-isopropyl-10H-phenothiazine HH-IPT (1.0g, 4.2mmol, 1eq.), 30% hydrogen peroxide (12mL, 415mmol, 100eq.) and acetic acid (35.5mL, 622.5mmol, 150eq.) Dissolve in chloroform solution, reflux and stir at 70°C for 4h.Stop the reaction, and after the reaction solution drops to room temperature, add an appropriate amount of 1M dilute hydrochloric acid to the reaction solution, extract the reaction solution with dichloromethane, collect the organic phase, dry it with anhydrous Na 2 SO 4 , and spin dry toobtainthecrudeproduct .Using dichloromethane:petroleum ether (1:1) as the eluent, the crude product was separated by silica gel column chromatography, and recrystallized from methanol to obtain a white solid HH-IPTOO (1.0g, 84.7%)
References:
CN115611826,2023,A Location in patent:Paragraph 0069-0075