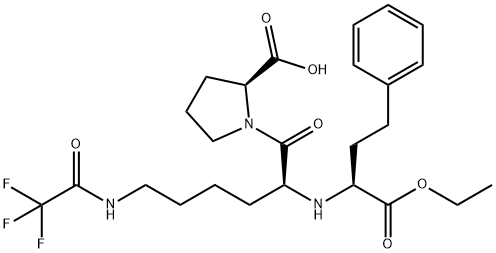
N2-1[(1S)-Ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysyl-L-proline synthesis
- Product Name:N2-1[(1S)-Ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysyl-L-proline
- CAS Number:103300-91-0
- Molecular formula:C25H34F3N3O6
- Molecular Weight:529.55
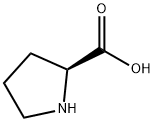
147-85-3
975 suppliers
$6.00/25g
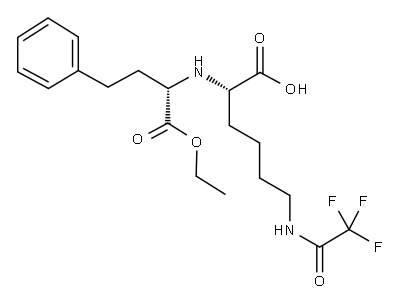
116169-90-5
151 suppliers
$7.00/5g
![N2-1[(1S)-Ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysyl-L-proline](/CAS/GIF/103300-91-0.gif)
103300-91-0
95 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
Stage #1:L-proline with tetrabutylammonium carbonate in ethanol at 50 - 60; for 0.166667 h;
Stage #2:N2-[1-(S)-ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysine with 1-hydroxy-pyrrolidine-2,5-dione;dicyclohexyl-carbodiimide in tetrahydrofuran at 0 - 30; for 9 h;Reagent/catalyst;Temperature;
Steps:
1-8 Example 7
Add 26g of L-valine to the reaction flask,55.4gTetrabutylammonium carbonateAnd 900mL of 95% ethanol, after stirring for 10 minutes,The temperature is raised to 50 to 60 ° C, and the solvent is evaporated under reduced pressure, and the mixture is cooled to room temperature.Then, 600 mL of tetrahydrofuran was added, and after stirring for 10 minutes, it was used as an L-valine ammonium salt solution.Add 80g to the reaction bottleLinoprilHydride,500mL of tetrahydrofuran (THF, succinic anhydride mass content of 18.4ppm),25g N-hydroxysuccinimide (NHS, succinic anhydride,The sum of the mass content of succinic acid and succinimide is 0.58%).Stir and dissolve, cool to 0 ~ 5 ° C, add 43g DCC,The above L-valine complex ammonium salt solution is slowly added dropwise at a temperature of 0 to 5 ° C.The temperature was raised to 20 to 30 ° C, the reaction was stirred for 9 hours, and filtered.The filtrate was distilled under reduced pressure until the solvent was completely distilled off, and after cooling to room temperature,Add 300 ml of water and adjust the pH of the solution to 4-5 with a sulfuric acid solution.Extracted with dichloromethane, and the organic phase is concentrated to obtain lisinopril condensate (III).Then add 500 mL of water and stir for 15 minutes.Slowly add 5 mol/L NaOH aqueous solution to the pH of the solution to 12-13.The temperature is raised to 40 to 45 ° C and stirred for 10 to 12 hours. After the reaction is completed,Cool to room temperature and adjust the pH to 7.5-8.5 with hydrochloric acid solution.The lisinopril solution was obtained (HPLC detection of N6-(3-carboxypropanoyl) lisinopril (I) impurity content was 0.02%),The lisinopril solution was transferred to a nanofiltration circulating bottle containing a 1.0 nm nanofiltration membrane for further purification, and concentrated under reduced pressure at 50 to 60 ° C to a volume of 120 mL.Recrystallization from 850 mL of 95% ethanol gave lisinopril 69.0 g.Yield: 92%(HPLC detection of N6-(3-carboxypropanoyl) lisinopril (I) impurity content was 0.01%).
References:
Zhejiang Huahai Pharmaceutical Co., Ltd.;Zhejiang Huahai Licheng Pharmaceutical Co., Ltd.;Lin Jinsheng;Wang Anyu;Zheng Yang;Wang Jichao;Hu Jiaxing;Fang Yuling;Zhu Wenquan;Chen Wenbin;Li Min CN109705010, 2019, A Location in patent:Paragraph 0045-0074
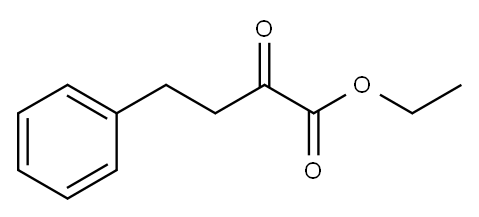
64920-29-2
248 suppliers
$10.00/1g

103300-89-6
63 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![N2-1[(1S)-Ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysyl-L-proline](/CAS/GIF/103300-91-0.gif)
103300-91-0
95 suppliers
$45.00/10mg