4-{6-Chloroimidazo[1,2-b]pyridazin-3-yl}benzonitrile synthesis
- Product Name:4-{6-Chloroimidazo[1,2-b]pyridazin-3-yl}benzonitrile
- CAS Number:1082604-63-4
- Molecular formula:C13H7ClN4
- Molecular Weight:254.67
![6-CHLORO-3-IODOIMIDAZO[1,2-B]PYRIDAZINE](/CAS/GIF/923595-49-7.gif)
923595-49-7
96 suppliers
$23.00/100mg
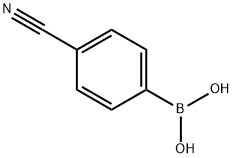
126747-14-6
397 suppliers
$6.00/1g
![4-{6-Chloroimidazo[1,2-b]pyridazin-3-yl}benzonitrile](/CAS/20210111/GIF/1082604-63-4.gif)
1082604-63-4
3 suppliers
inquiry
Yield:1082604-63-4 98%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in water;N,N-dimethyl-formamide at 90; for 18 h;Inert atmosphere;
Steps:
1
Step 1 : To a solution of 6-chloro-3-iodoimidazo[l,2-b]pyridazine (1.00 g, 3.58 mmol) in a mixture of DMF (20 mL) and water (1 mL) under inert atmosphere, were added Na2C03 (759 mg, 7.16 mmol), 4-cyanophenylboronic acid (885 mg, 5.37 mmol) and Pd(PPh3)4 (414 mg, 0.358 mmol). The resulting mixture was stirred at 90 °C for 18 h and quenched with ice water. The precipitate was isolated by filtration and dried in vacuo to afford 4-(6- chloroimidazo[l,2-6]pyridazin-3-yl)benzonitrile (897 mg, 98%) as a yellow solid. 1H NMR FontWeight="Bold" FontSize="10" --·.·· (400 MHz, CDC13) δ 8.24-8.21 (m, 2H), 8.18 (s, 1H), 8.02 (d, J= 9.4 Hz, 1H), 7.81-7.79 (m, 2H), 7.45 (d, J = 9.4 Hz, lH); MS (ESI) m/z 255 [C13H7C1N4 + H]+.
References:
WO2013/147711,2013,A1 Location in patent:Page/Page column 131
![3-Bromo-6-chloroimidazo[1,2-b]pyridazine](/CAS/GIF/13526-66-4.gif)
13526-66-4
204 suppliers
$15.00/1g

126747-14-6
397 suppliers
$6.00/1g
![4-{6-Chloroimidazo[1,2-b]pyridazin-3-yl}benzonitrile](/CAS/20210111/GIF/1082604-63-4.gif)
1082604-63-4
3 suppliers
inquiry
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)