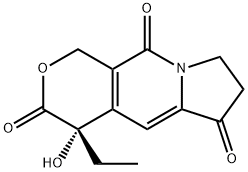
(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione synthesis
- Product Name: (S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione
- CAS Number:110351-94-5
- Molecular formula:C13H13NO5
- Molecular Weight:263.25
![Synthesis of (S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/NewsImg/2022-07-14/6379343161441863966536659.jpg)
![CHUGAI-01
(4'S)-4'-ethyl-1',4',7',8'-tetrahydro-4'-hydroxy-3'H,10'H-spiro[1,3-dioxolane-2,6'-pyrano[3,4-f]indolizine]-3',10'-dione](/CAS/20200611/GIF/110351-93-4.gif)
110351-93-4
13 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/CAS/GIF/110351-94-5.gif)
110351-94-5
199 suppliers
$295.00/250MG
Yield:110351-94-5 93%
Reaction Conditions:
with trifluoroacetic acid at 20; for 6 h;
Steps:
30.7 Step (7):(5 ′S) -1,5-dioxo- (5′-ethyl-5′-hydroxy-2′H, 5′H, 6′H-6-oxopyran)-[3 ′, 4 ′, f] -Δ6 (8) -tetrahydroindolizine(Intermediate M) Preparation
The intermediate M14 (1.0 g, 3.26 mmol) was dissolved in trifluoroacetic acid (30 ml),The reaction was stirred at room temperature for 6 hours, and was concentrated by rotary evaporation to obtain a dark brown oily liquid.Purification by silica gel column chromatography (dichloromethane: ethyl acetate = 4: 1) gave 0.88 g of a pale yellow solid with a yield of 93%.
References:
CN110590796,2019,A Location in patent:Paragraph 0223-0227; 0250-0253
![(S)-4-Ethyl-4,6-dihydroxy-3,10-dioxo-3,4,8,10-tetrahydro-1H-pyrano[3,4-f]indolizine-7-carboxylic acid tert-butyl ester](/CAS/GIF/183434-04-0.gif)
183434-04-0
1 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/CAS/GIF/110351-94-5.gif)
110351-94-5
199 suppliers
$295.00/250MG

165674-36-2
1 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/CAS/GIF/110351-94-5.gif)
110351-94-5
199 suppliers
$295.00/250MG
![Spiro[1,3-dioxolane-2,6'(3'H)-[1H]pyrano[3,4-f]indolizine]-3',10'(4'H)-dione, 4'-ethyl-7',8'-dihydro-4'-hydroxy-](/CAS/20200611/GIF/102978-41-6.gif)
102978-41-6
5 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/CAS/GIF/110351-94-5.gif)
110351-94-5
199 suppliers
$295.00/250MG
![(S)-α-ethyl-α-hydroxy-1,1-(ethylenedioxy)-6-hydroxymethyl-5-oxo-1,2,3,5-tetrahydroindolizine-7-[N-(1S)-1-phenylethyl]acetamide](/CAS/20211123/GIF/110314-10-8.gif)
110314-10-8
4 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-7,8-dihydro-1h-pyrano[3,4-f]indolizine-3,6,10(4h)-trione](/CAS/GIF/110351-94-5.gif)
110351-94-5
199 suppliers
$295.00/250MG