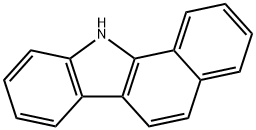
11H-BENZO[A]CARBAZOLE synthesis
- Product Name:11H-BENZO[A]CARBAZOLE
- CAS Number:239-01-0
- Molecular formula:C16H11N
- Molecular Weight:217.27
![6,11-dihydro-5H-benzo[a]carbazole](/CAS/GIF/21064-49-3.gif)
21064-49-3
15 suppliers
$65.00/100mg
![11H-BENZO[A]CARBAZOLE](/CAS/GIF/239-01-0.gif)
239-01-0
114 suppliers
$70.00/50mg
Yield:239-01-0 91%
Reaction Conditions:
with copper(II) choride dihydrate in dimethyl sulfoxide at 20 - 120;Reagent/catalyst;
Steps:
General procedure for the synthesis of benzocarbazole, pyridazine-3-one and indole
General procedure: In 50 ml round bottom flask, CuCl2*2H2O (4.5 mmol) thoroughly dissolved in 10mL DMSO. To this solution, dihydrobenzo[a]carbazole (2.2 mmol) was added and stirred for 30 min at room temperature. The resulting mixture was heated at 120 C through open vessel. The progress of the reaction was monitored by TLC. After completion ofthe reaction, the mixture was quenched by few drops of con. HCl and poured in ice cold water. The product was isolated. Filter and recrystalized by methanol. 11H-benzo[a]carbazole (3a): Yield 91%, m.p.: 230-231 C, IR ( cm1): 3030, 1600,1587; 1H NMR (300 MHz, CDCl3): d 8.13 (d, J8.1 Hz, 1H), 8.01 (d, J8.1 Hz, 1H),7.62 (d, J7.8 Hz, 1H), 7.45-7.28 (m, 7H), 4.28 (s, 3H); 13C NMR (100 MHz, CDCl3):140.4, 136.5, 134.8, 133.5, 130.1, 125.5, 125.2, 123.9, 123.8, 121.1, 120.6, 120.1, 119.7,118.9, 119.1, 111.0, 44.6; HRMS (ES) m/z232.1004 calcd for C17H13N [MH],found 232.1007
References:
Humne, Vivek T.;Ulhe, Avinash G. [Synthetic Communications,2020] Location in patent:supporting information

94064-83-2
1 suppliers
inquiry
![11H-BENZO[A]CARBAZOLE](/CAS/GIF/239-01-0.gif)
239-01-0
114 suppliers
$70.00/50mg

74886-75-2
1 suppliers
inquiry
![11H-BENZO[A]CARBAZOLE](/CAS/GIF/239-01-0.gif)
239-01-0
114 suppliers
$70.00/50mg
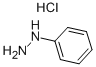
59-88-1
353 suppliers
$15.00/10g
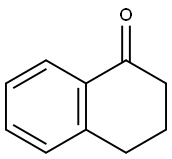
529-34-0
482 suppliers
$6.00/10g
![11H-BENZO[A]CARBAZOLE](/CAS/GIF/239-01-0.gif)
239-01-0
114 suppliers
$70.00/50mg

71221-26-6
4 suppliers
inquiry
![11H-BENZO[A]CARBAZOLE](/CAS/GIF/239-01-0.gif)
239-01-0
114 suppliers
$70.00/50mg
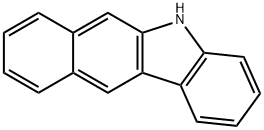
243-28-7
77 suppliers
$56.00/100mg