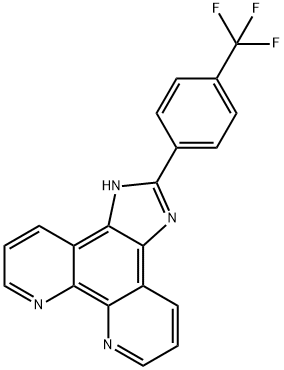
2-(4-trifluoroMethylphenyl)iMidazole[4,5f][1,10]phenanthroline synthesis
- Product Name:2-(4-trifluoroMethylphenyl)iMidazole[4,5f][1,10]phenanthroline
- CAS Number:1233850-88-8
- Molecular formula:C20H11F3N4
- Molecular Weight:364.32
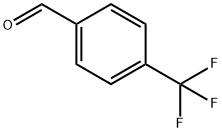
455-19-6
408 suppliers
$6.00/5g
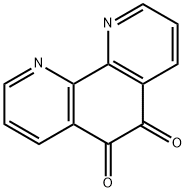
27318-90-7
276 suppliers
$7.00/250mg
![2-(4-trifluoroMethylphenyl)iMidazole[4,5f][1,10]phenanthroline](/CAS/20210111/GIF/1233850-88-8.gif)
1233850-88-8
0 suppliers
inquiry
Yield:1233850-88-8 67.7%
Reaction Conditions:
with ammonium acetate;acetic acid at 110; for 4 h;
Steps:
Synthesis of 1a, 2a, 3a, 4a, 5a, 6a and 7a
General procedure: A solution containing 1,10-phenanthroline-5,6-dione (1.6 mmol, 347 mg), substituted benzaldehyde (1.6 mmol), 20 ml of HAc and NH4Ac (33 mmol, 2.53 g), was heated at 110 °C under reflux for 4 h. Then, 20 ml of water was added and the pH value was adjusted to 7.0 at room temperature. The solution was filtered and dried in vacuum to obtain a yellow precipitate. The product was purified in a silica gel column by using ethanol as eluent. 1a: yield 78.4%; mp. 217-219 °C, ESI-MS (in MeOH): m/z: 219.1, ([M + H]), 438.1,([M + 2H]2+). 2a: yield 79.5%; mp. 226-228 °C, ESI-MS (in MeOH): m/z: 339.15, ([M + H]+), 678.1, ([M + 2H]2+). 3a: yield 64.4%; mp. 262-265 °C, ESI-MS (in MeOH): m/z: 325.1, ([M + H]+), 650.3, ([M + 2H]2+). 4a: yield 75.7%; mp. 234-236 °C, ESI-MS (in MeOH): m/z: 311.1, ([M + H]+). 5a: yield 67.1%; mp. 232-235 °C, ESI-MS (in MeOH): m/z: 339.1, ([M + H]+). 6a: yield 67.7%; mp. 280-283 °C, ESI-MS (in MeOH): m/z: 363.1, ([M + H]+), 726.1, ([M + 2H]2+). 7a: yield 63.4%; mp. 269-273 °C, ESI-MS (in MeOH): m/z: 375.2, ([M + H]+), 750, ([M 2H]2+), 772.8, ([M + H + Na]2+).
References:
Wu, Qiong;Fan, Cundong;Chen, Tianfeng;Liu, Chaoran;Mei, Wenjie;Chen, Sidong;Wang, Baoguo;Chen, Yunyun;Zheng, Wenjie [European Journal of Medicinal Chemistry,2013,vol. 63,p. 57 - 63]
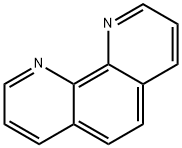
66-71-7
374 suppliers
$6.00/5g
![2-(4-trifluoroMethylphenyl)iMidazole[4,5f][1,10]phenanthroline](/CAS/20210111/GIF/1233850-88-8.gif)
1233850-88-8
0 suppliers
inquiry