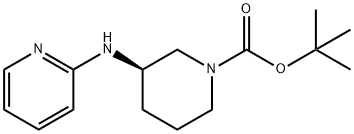
(R)-tert-Butyl 3-(pyridin-2-ylamino)piperidine-1-carboxylate synthesis
- Product Name:(R)-tert-Butyl 3-(pyridin-2-ylamino)piperidine-1-carboxylate
- CAS Number:1255533-88-0
- Molecular formula:C15H23N3O2
- Molecular Weight:277.36
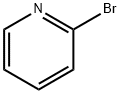
109-04-6
522 suppliers
$6.00/25g
188111-79-7
360 suppliers
$6.00/1g

1255533-88-0
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium tert-butylate;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 90; for 16 h;Inert atmosphere;
Steps:
1 Step 1: Synthesis of Compound WXOO6-1
10162] WXBB-3-1 (1.27 g, 6.33 mmol, 1.00 eq) and2-bromopyridine (1.00 g, 6.33 mmol, 602.41 pL, 1.00 eq) were dissolved in methylbenzene (20.00 mE). The mixture was placed in a 50 mE single-mouth round-bottom flask, which was then added with tris(dibenzylideneacetone)dipalladium (579.65 mg, 633.00 jimol, 0.10 eq), (±)-2,2’-bis- diphenyl phosphino- 1, 1’-binaphthyl(59 1.23 mg, 949.50 jimol, 0.15 eq) and potassium tert-butoxide (1.48 g, 13.17 mmol, 2.08 eq). The mixture was stirred at 90° C. for 16 h under nitrogen condition. Afier reaction, the mixture was concentrated, then was added with water (30 mE) and extracted with dichloromethane (20 mE*3), The organic phase was dried with sodium sulfate, filtered, and concentrated. The crude was purified by chromatographic column (petroleum ether:ethyl acetate=30: 1-1:1), and further purifled by preparative HPEC to obtain WXOO6-1. ‘H NMR (400 MHz, CDC13) ?ppm: 8.07 (d, J=4 Hz, 1H), 7.44-7.40 (m, 1H), 6.59-6.56 (m, 1H), 6.43 (d, J=8.4 Hz, 1H), 4.52 (br, 1H), 4.00-2.8 (brm, 4H), 2.01-1.97 (m, 1H), 1.77-1.71 (m, 1H), 1.60-1.56 (m, 2H), 1.43 (s, 9H). MS mlz: 278.1 [M+H].
References:
US2018/305346,2018,A1 Location in patent:Paragraph 0160; 0161; 0162