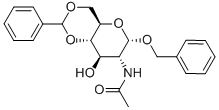
BENZYL 2-ACETAMIDO-4,6-O-BENZYLIDENE-2-DEOXY-ALPHA-D-GLUCOPYRANOSIDE synthesis
- Product Name:BENZYL 2-ACETAMIDO-4,6-O-BENZYLIDENE-2-DEOXY-ALPHA-D-GLUCOPYRANOSIDE
- CAS Number:13343-63-0
- Molecular formula:C22H25NO6
- Molecular Weight:399.44
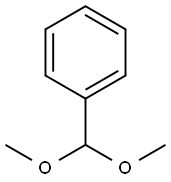
1125-88-8
345 suppliers
$6.00/25g

13343-62-9
90 suppliers
$26.00/1g

13343-63-0
103 suppliers
$59.70/500mg
Yield: 95%
Reaction Conditions:
with toluene-4-sulfonic acid in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
Compound 11 (0.412 g, 1.32 mmol) was dissolved in DMF (20 mL) followedby the addition of benzaldehyde dimethyl acetal (0.6 mL, 3.97 mmol) and acatalytic amount of p-toluenesulfonic acid (0.123 g, 0.53 mmol). The reactionmixture was stirred for 3 h. DMF was removed in vacuo and the resultantwhite residue was suspended in saturated sodium bicarbonate solution. Uponfiltration, the residue was washed several times with hexanes/EtOAc/CH2Cl2(4/1/0.5) solvent system then dried under vacuum to afford compound 12 in95% yield (0.5 g). Comparison of 1H NMR with reported literature values confirmedthe identity of compound 12.[32] 1H NMR (600 MHz, CDCl3) δ 1.82 (s, 3H,CH3), 3.59 (t, 1H, J3 = 9 Hz, H-6), 3.75 (t, 1H, J3 = 10.2 Hz, H-6’), 3.84-3.91(m, 1H, H-5), 3.92-3.95 (m, 1H, H-4), 4.23-4.26 (m, 2H, H-2, H-3), 4.93 (d, 1H,J3 = 12 Hz, CH2Ph), 4.74 (d, 1H, J3 = 12 Hz, CH2Ph), 4.93 (d, 1H, J3 = 3.5 Hz,H-1), 5.57 (s, 1H, CHPh), 5.82 (d, 1H, J3 = 8.4 Hz, CONH), 7.33-7.51 (m, 10H,aromatic).
References:
Wasonga, Gilbert;Tatara, Yota;Kakizaki, Ikuko;Huang, Xuefei [Journal of Carbohydrate Chemistry,2013,vol. 32,# 5-6,p. 392 - 409]

100-52-7
948 suppliers
$17.30/2g

13343-62-9
90 suppliers
$26.00/1g

13343-63-0
103 suppliers
$59.70/500mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13343-63-0
103 suppliers
$59.70/500mg

13347-83-6
0 suppliers
inquiry

108-24-7
5 suppliers
$14.00/250ML

13343-63-0
103 suppliers
$59.70/500mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13343-63-0
103 suppliers
$59.70/500mg