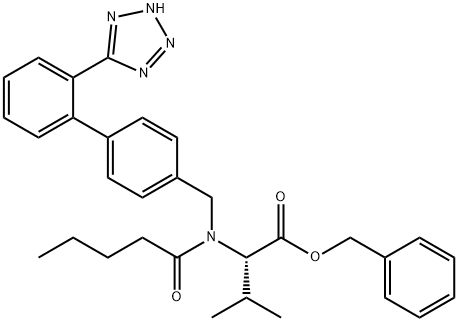
N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester synthesis
- Product Name:N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester
- CAS Number:137863-20-8
- Molecular formula:C31H35N5O3
- Molecular Weight:525.64
676129-93-4
13 suppliers
$139.80/10mg
638-29-9
261 suppliers
$14.14/1gm:
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
104 suppliers
$70.00/5mg
Yield:> 99.8 % ee
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in toluene at 0 - 50; for 0.5 - 1 h;
Steps:
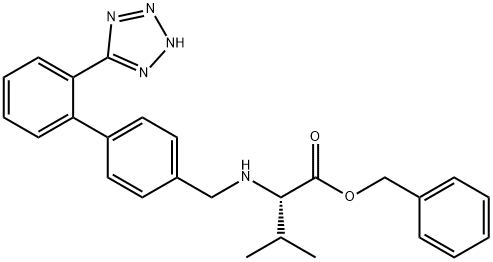
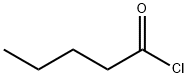
2.b; 7.b b) Preparation of Preparation of (S)-3-Methyl-{2-pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl-methyl]- [AMINOT-BUTYRIC] acid amino}-butyric acid benzylester
The solution of [(S)-3-METHYL-2-{[2'-(1 H-TETRAZOL-5-YL)-BIPHENYL-4-YL-METHYL]-AMINO}-BUTYRIC..] acid benzylester (approximately 7.0 g, 16.0 [MINOT)] in toluene (35 [ML)] from the previous step. is diluted with toluene (35 [ML).] The clear [ SOLUTION IS. COOLED TO 0-5°C] under [ANHYDROUS CONDI-] tions, and [N-ETHYL-DIISOPROPYLAMINE] (6.1 [MI,.] [35. 2 MOI)] and [VALEROYLCHLORIDE] (4.1 [M), 33. 6] mmol) are added at this temperature. The reaction. mixture is heated to [50°C] within 30 min and agitated at [50°C] for approximately 1 h and-after completion of the reaction-quenched by addition of methanol (10 ml) at [50°C.] The clear solution is stirred for approximately 30 min at [50°C] and finally cooled to RT. Water (30 ml) is added and the resulting two-phase system is adjusted to pH 2 by addition of hydrochloric acid 2.0 M (approximately 11 [MI.'22] [MMOL).] The organic phase is separated, extracted with water (30 [ML)] and concentrated at [50°C] in vacuo to approximately 50 % of the original volume by destillation (water and methanol are [AZEOTROPICALLY] removed). The resulting concentrate in toluene (40 [ML)] is seeded at [40°C] in order to start the crystallization and agitated at this temperature for approximately 1 hour (h). The suspension is gradually cooled to [0°C] within 6-10 h. The solid is separated by filtration, washed with cold toluene (30 [ML)] and dried in vacuo at [50°C] to give (S)-3-methyl-{2-pentanoyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl-methyl]-amino}-butyric acid benzylester. Melting point: [115-116°C] Enantiomeric excess (by HPLC): ee > 99.8 % The solution of [(S)-3-METHYL-2-{[2'-(1 H-TETRAZOL-5-YL)-BIPHENYL-4-YL-METHYL]-AMINO}-BUTYRIC] acid benzylester in toluene (approximately 80 g, 48-50 [MMOL)] from the previous step is di- luted with toluene (85 [ML).] Under anhydrous conditions, N-ethyl-diisopropylamine (24. 0 ml, 140 mol) and [VALEROYLCHLORIDE (17. 3 ML,] 140 [MMOL)] are slowly added at [20°C] internal tempe- rature. The reaction mixture is agitated for approximately 30 min and-after completion of the transformation-quenched by addition of methanol (31 ml) at [20°C.] The clear solution is agitated for 30 min at [20°C,] then water (78 [ML)] is added and the resulting two-phase system is adjusted to pH 2 by addition of 2.0 M'hydrochloric acid (approximately 10 ml, 20 mmol). The organic phase is separated, extracted with water (78 [MT)] and concentrated at [50°C] in vacuo to approximately [50%] of the original volume by distillation (water and methanol are azeotropically removed). The resulting concentrate in toluene [(No. 94 G) ] is seeded at [40°C] in order to initiate the crystallization and agitated at this temperature for approximately 1 h. The suspension is gradually cooled to [0°C] within 6-10 h. The solid is separated by filtration, wa- shed with cold [TOLUENE] (60 ml) and dried in vacuo at [50°C] to give [(S)-3-METHYL- {2-PENTANOYL-] [[2'- (1 H-TETRAZOL-5-YL)-BIPHENYL-4-YL-METHYL]-AMINO}-BUTYRIC] acid benzylester.. Melting point: [115-116°C.] Enantiomeric excess (by [HPLC) :] ee > 99.8 %.
References:
WO2004/26847,2004,A1 Location in patent:Page 26; 32-33
![N-[(2′-(1-Triphenylmethyl-Tetrazole-5-Yl)Biphenyl-4-Yl]-Methyl]-N-Valeryl-L- Valine Benzyl Ester](/CAS/20200611/GIF/137864-44-9.gif)
137864-44-9
3 suppliers
inquiry
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
104 suppliers
$70.00/5mg
![(S)-Benzyl 2-(N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoate](/CAS/20180703/GIF/137864-22-3.gif)
137864-22-3
5 suppliers
inquiry
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
104 suppliers
$70.00/5mg

67-56-1
731 suppliers
$9.00/25ml
![N-[(2′-(1-Triphenylmethyl-Tetrazole-5-Yl)Biphenyl-4-Yl]-Methyl]-N-Valeryl-L- Valine Benzyl Ester](/CAS/20200611/GIF/137864-44-9.gif)
137864-44-9
3 suppliers
inquiry
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
104 suppliers
$70.00/5mg

596-31-6
79 suppliers
$110.00/100mg
![L-Valine, N-(1-oxopentyl)-N-[[2'-[1-(tributylstannyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-, phenylmethyl ester](/CAS/20210305/GIF/1315376-91-0.gif)
1315376-91-0
0 suppliers
inquiry
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
104 suppliers
$70.00/5mg