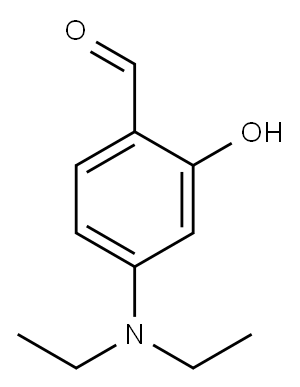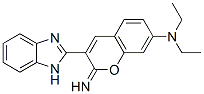
3-(1H-benzoimidazol-2-yl)-N,N-diethyl-2-imino-chromen-7-amine synthesis
- Product Name:3-(1H-benzoimidazol-2-yl)-N,N-diethyl-2-imino-chromen-7-amine
- CAS Number:17754-91-5
- Molecular formula:C20H20N4O
- Molecular Weight:332.41
Yield:17754-91-5 82%
Reaction Conditions:
Stage #1: 1H-benzimidazol-2-acetonitrilewith piperidine in methanol at 20; for 0.5 h;
Stage #2: 4-(Diethylamino)salicylaldehyde in methanol at 20; for 5 h;
Steps:
Synthesis of 3-Benzimidazole Iminocoumarin 3
To a solution of 2-cyano methyl benzimidazole 1 (0.55 g,2.8 mmol), piperidine (0.1 mL, 1.4 mmol) was added in dry methanol (50 mL), and the solution was stirred at room temperature for 30 min. Then 4-(N, N-diethyl amino)-2-hydroxybenzaldehyde (0.49 g, 2.8 mmol) was added. The mixture was stirred for 5 h at room temperature, and the precipitate was collected by filtration, washed with dry methanol,and dried under high vacuum to obtain a yellow solid of 3-benzimidazole iminocoumarin 3. Yield = 82 %, m.p.238 °C(lit.239 °C)
References:
Chemate, Santosh B.;Sekar, Nagaiyan [Journal of Fluorescence,2015,vol. 25,# 6,p. 1615 - 1628]
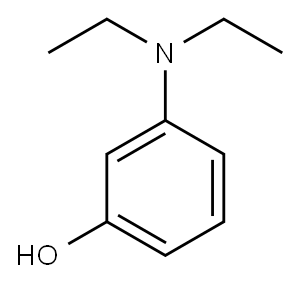
91-68-9
282 suppliers
$9.00/25g

17754-91-5
3 suppliers
inquiry
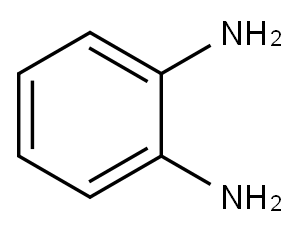
95-54-5
486 suppliers
$9.00/1g

17754-91-5
3 suppliers
inquiry