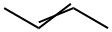2,3,4-TRIMETHYLPENTANE synthesis
- Product Name:2,3,4-TRIMETHYLPENTANE
- CAS Number:565-75-3
- Molecular formula:C8H18
- Molecular Weight:114.23

67-56-1
733 suppliers
$7.29/5ml-f
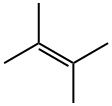
563-79-1
135 suppliers
$29.00/5mL

74-88-4
341 suppliers
$15.00/10g
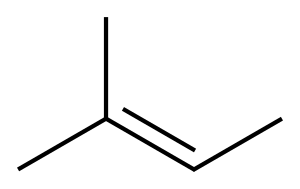
513-35-9
173 suppliers
$12.00/25g

565-75-3
70 suppliers
$52.00/5g
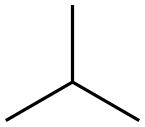
75-28-5
122 suppliers
$40.00/1g
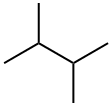
79-29-8
129 suppliers
$20.00/1g

594-56-9
54 suppliers
$39.00/1mL
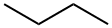
106-97-8
102 suppliers
$36.00/1mL
Yield:-
Reaction Conditions:
diiodidotetracarbonylruthenium(II) at 160; for 3 h;Sealed tube;
Steps:
1
The [Ru(CO)4I2], 2,3-dimethyl-2-butene and then the methanol were weighed into a 15ml ACE glass pressure tube. The tube was then agitated with a spatula and shaken to dissolve the [Ru(CO)4I2], most of which did not dissolved. No heat was evolved. The methyl iodide was added and the tube sealed. The contents of the tube appeared to have separated into three layers, the bottom layer contained orange solid, whilst the top layer was orange in colour. The middle layer was yellow in colour and appeared to contain suspended solid. The tube was encased in a mesh, placed in a metal beaker and then placed in an oven at 160°C for 3 hours. On cooling, the tube contained a lot of red solid and a clear orange liquid layer. The tube was cooled to room temperature and when opened, it released a lot of pressured gas. The contents continued to bubble so the top was left off for the next 20 hours until the bubbling had stopped. 50µl of cyclohexane was then added to act as an internal standard, the contents were shaken and allowed to settle. An aliquot (50µl) was removed and diluted with deuterated trichloromethane (250µl), CDCl3, for gas chromatographic (GC) analysis, the results of which are set out in Table 1. It was observed that the product of the reaction is a hydrocarbon, triptene. The yield of triptene based upon 2,3-dimethylbut-2-ene fed to the reactor was 23.3%, with a selectivity based upon 2,3-dimethylbut-2-ene converted of 30.3%. Negligible triptane (identified by NMR by comparison with a standards containing triptane and triptene and GC-MS - fragmentation pattern in the mass spectrum and matching against library spectra) was produced. The low mass accountability was attributed to loss of light materials on opening the sample. Analysis by GC-MS showed the presence of dimethyl butanols, trimethyl-2-butanol and 2,2,3-trimethylmethoxybutane; This example shows the production of a hydrocarbon by reacting, in a reactor, methanol with an olefin in the presence of methyl iodide and a ruthenium carbonyl halide compound. In particular, this example shows the production of a hydrocarbon (triptene) by reacting, in a reactor, methanol with an olefin (2,3-dimethylbut-2-ene) in the presence of methyl iodide and a ruthenium carbonyl halide compound having an empirical formula [Ru(CO)4I2].
References:
BP OIL INTERNATIONAL LIMITED EP1900714, 2008, A1 Location in patent:Page/Page column 4-6

565-77-5
32 suppliers
$15.00/0.1mL

565-75-3
70 suppliers
$52.00/5g

75-28-5
122 suppliers
$40.00/1g
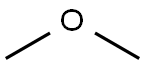
115-10-6
93 suppliers
$75.00/25 g
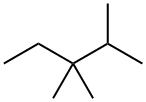
560-21-4
25 suppliers
inquiry

565-75-3
70 suppliers
$52.00/5g

540-84-1
369 suppliers
$16.00/25ML
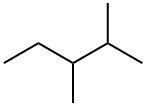
565-59-3
80 suppliers
$44.20/5g
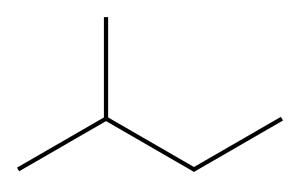
78-78-4
265 suppliers
$20.00/25ML
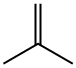
115-11-7
228 suppliers
$45.00/25 g

560-21-4
25 suppliers
inquiry

565-75-3
70 suppliers
$52.00/5g

540-84-1
369 suppliers
$16.00/25ML

78-78-4
265 suppliers
$20.00/25ML