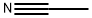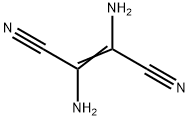
2,3-DIAMINOBUT-2-ENEDINITRILE synthesis
- Product Name:2,3-DIAMINOBUT-2-ENEDINITRILE
- CAS Number:18514-52-8
- Molecular formula:C4H4N4
- Molecular Weight:108.1
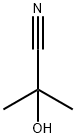
75-86-5
2 suppliers
$80.00/5g

18514-52-8
27 suppliers
inquiry
Yield: 88%
Reaction Conditions:
with sodium cyanide;methylthiol at 60; for 2 h;Autoclave;Temperature;Reagent/catalyst;
Steps:
2
In an autoclave, 10g of NaCN is added to 130 ml of acetone cyanohydrin. Then 6.54g methanethiol is added. The solution is then stirred at 60 °C. for 2 hours. The Solution changes is orange. The excess acetone cyanohydrin, then, is neutralized by adding soda. The solution is then filtered and evaporated to give an orange-yellow solid (amount recovered = 33. 7g corresponding to a yield = 88%).
References:
ARKEMA FRANCE;SCHMIDT, GREGORY JP2015/528803, 2015, A Location in patent:Paragraph 0030
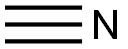
74-90-8
4 suppliers
inquiry

18514-52-8
27 suppliers
inquiry