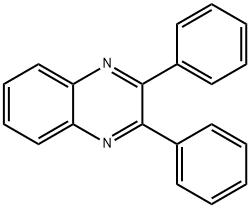
2,3-DIPHENYLQUINOXALINE synthesis
- Product Name:2,3-DIPHENYLQUINOXALINE
- CAS Number:1684-14-6
- Molecular formula:C20H14N2
- Molecular Weight:282.34
Yield: 92%
Reaction Conditions:
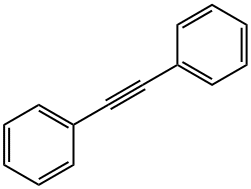
Stage #1:diphenyl acetylene with iodine in dimethyl sulfoxide at 130; for 24 h;
Stage #2:1,2-diamino-benzene in dimethyl sulfoxide at 20; for 1 h;Catalytic behavior;Temperature;
Steps:
General procedure for iodine-catalyzed one-pot annulation of alkynes and o-phenylenediamines.
General procedure: To a stirred solution of alkyne (0.2 mmol) in DMSO (2.0 mL) was added I2 (10.1 mg, 0.04 mmol). The solution was stirred at 130 oC for 24 h in air. Then the mixture was cooled to room temperature, and o-Phenylenediamine (0.3 mmol) was added. The solution was stirred at room temperature for 1 h. After completion, the solution was diluted with ethyl acetate, washed with H2O, dried over MgSO4, and concentrated in vacuo. The residue was purified by preparative thin-layer chromatography on silica gel with PE/EtOAc (15/1) as an eluent to give quinoxaline 3. 2,3-Diphenylquinoxaline (3aa).[2] White solid, mp: 120-122 oC; 51.9 mg (92% yield); 1H NMR (400 MHz, CDCl3) δ 8.17 (dd, J = 6.4, 3.4 Hz, 2H), 7.75 (dd, J = 6.4, 3.4 Hz, 2H), 7.52 (dd, J = 7.7, 1.8 Hz, 4H), 7.39-7.28 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 128.34, 128.88, 129.29, 129.94, 130.02, 139.18, 141.32, 153.54.
References:
Zi, Jing;Gu, Da-Wei;Zhang, Yan;Hu, Zhe-Yao;Zhang, Xing-Quan;Guo, Xun-Xiang [Synthetic Communications,2018,vol. 48,# 8,p. 915 - 920] Location in patent:supporting information
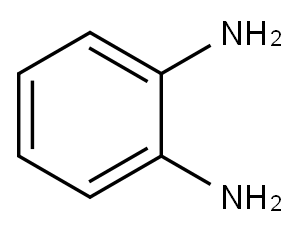
95-54-5
488 suppliers
$9.00/1g

7338-94-5
31 suppliers
$220.00/100mg

1684-14-6
109 suppliers
$8.00/5g