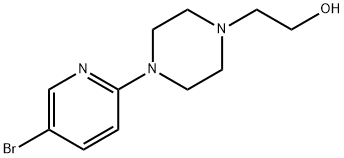
2-[4-(5-Bromo-2-pyridinyl)-1-piperazinyl]ethanol synthesis
- Product Name:2-[4-(5-Bromo-2-pyridinyl)-1-piperazinyl]ethanol
- CAS Number:364794-69-4
- Molecular formula:C11H16BrN3O
- Molecular Weight:286.17
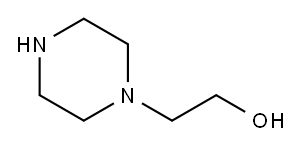
103-76-4
504 suppliers
$6.00/25g
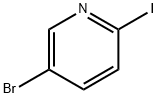
223463-13-6
366 suppliers
$5.00/1g
![2-[4-(5-Bromo-2-pyridinyl)-1-piperazinyl]ethanol](/CAS/GIF/364794-69-4.gif)
364794-69-4
9 suppliers
$108.00/500mg
Yield:364794-69-4 26%
Reaction Conditions:
in acetonitrile; for 24 h;Heating / reflux;
Steps:
115
Example 115. 2-f4-(5-Bromo-pyridin-2-vI)-piperazin-l-yll-ethanoI (63); [0291] A mixture of 5-bromo-2-iodo-pyridine (5.0 g, 18 mmol) and 2-piperazin-l-yl- ethanol (5.0 g, 39 mmol) in acetonitrile (40 mL) was heated at reflux for 1 d. The mixture was allowed to cooled to room temperature, poured into water and extracted with EtOAc. The organic layer was washed with water, brine, and dried over MgSθ4 and filtered. The filtrate was concentrated and the residue purified by flash chromatography on silica gel (5% MeOH/DCM to 10% MeOH/DCM) to afford the title compound (1.3 g, 26%) as a white solid.
References:
WO2006/101977,2006,A2 Location in patent:Page/Page column 102