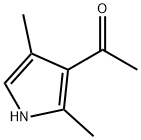
2,4-Dimethylpyrrole synthesis
- Product Name:2,4-Dimethylpyrrole
- CAS Number:625-82-1
- Molecular formula:C6H9N
- Molecular Weight:95.14

7073-36-1
90 suppliers
$14.00/1g

625-82-1
263 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
in tetrahydrofuran
Steps:
47 Starting compound II: 2,4-dimethylpyrrole (0.21 mL, 2.0 mmol)
Starting compound II: 2,4-dimethylpyrrole (0.21 mL, 2.0 mmol) Starting compound III: 2-chloro-4-nitrobenzoyl chloride (0.48 g, 2.2 mmol) in THF (1 mL)
References:
Havez, Sophie Elisabeth US2003/73832, 2003, A1
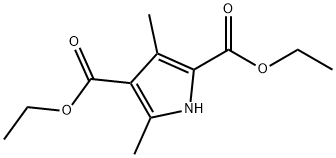
2436-79-5
192 suppliers
$16.00/1 / G

625-82-1
263 suppliers
$9.00/1g
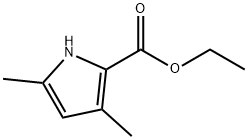
2199-44-2
145 suppliers
$8.00/1g
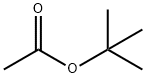
540-88-5
350 suppliers
$21.00/25mL

625-82-1
263 suppliers
$9.00/1g

28991-95-9
37 suppliers
$302.00/25g