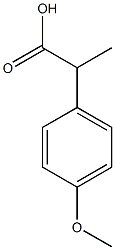
2-(4-methoxyphenyl)propanoic acid synthesis
- Product Name:2-(4-methoxyphenyl)propanoic acid
- CAS Number:942-54-1
- Molecular formula:C10H12O3
- Molecular Weight:180.2
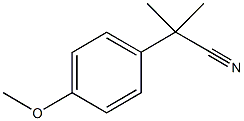
5351-07-5
44 suppliers
inquiry

942-54-1
43 suppliers
$50.00/100mg
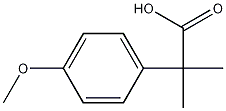
2955-46-6
48 suppliers
$45.00/25mg
Yield: 78% , 2%
Reaction Conditions:
Stage #1:2-(4-methoxy-phenyl)-2-methyl-propionitrile with potassium hydroxide in water;ethylene glycol at 20 - 160;
Stage #2: with hydrogenchloride in monoethylene glycol diethyl ether;water; pH=2
Steps:
D.2
A mixture of 2- (4-methoxyphenyl)-2-methylpropionitrile g, 2.04 mol), KOH (284.8 g, 5.08 mol), ethylene glycol (750 mL), and water (100 mL) was heated at 150-160 °C for 7 h in a 1L round-bottom flask equipped with a bump flask and fermentation lock, then allowed to cool and stand overnight. Heating was continued for an additional 7 hours, without any additional conversion being observed. The reaction was allowed to cool and poured into water (2 L), then acidified with stirring to pH 10-11 by addition of concentrated HCI (-250 mL). The resulting solution was extracted with isopropyl acetate (1x1 L, followed by 2x500 mL) and then filtered to remove a small quantity of a white precipitate. The reaction mixture was stirred vigorously and slowly acidified further to ca pH = 2 with concentrated HCl (-250 mL). The product started to precipitate at pH 6-7. The suspension was stirred for 30 minutes at ambient temperature then kept a refrigerator overnight. The mixture was filtered and the precipitate was washed with cold 1M HCl (500 mL), followed cold water (2x150 mL). The solid was dried under suction, follow by high vacuum (lyophilizer) overnight to give 2- (4-methoxyphenyl)-2-methylpropanoic (314 g, 80%) as pale yellow-orange crystals containing approximately 2.0 % of the mono-methyl impurity.
References:
AXYS PHARMACEUTICALS, INC. WO2005/121102, 2005, A2 Location in patent:Page/Page column 24-25

2901-41-9
0 suppliers
inquiry

942-54-1
43 suppliers
$50.00/100mg
637-69-4
224 suppliers
$8.00/1g

124-38-9
122 suppliers
$175.00/23402
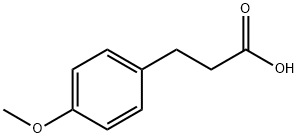
1929-29-9
229 suppliers
$8.00/5g

942-54-1
43 suppliers
$50.00/100mg

51747-42-3
1 suppliers
inquiry

942-54-1
43 suppliers
$50.00/100mg

124-38-9
122 suppliers
$175.00/23402

1538-89-2
4 suppliers
inquiry

942-54-1
43 suppliers
$50.00/100mg