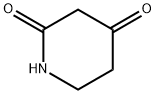
2,4-Piperadinedione synthesis
- Product Name:2,4-Piperadinedione
- CAS Number:50607-30-2
- Molecular formula:C5H7NO2
- Molecular Weight:113.11
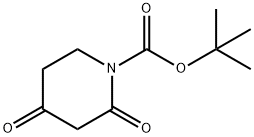
845267-78-9
166 suppliers
$10.00/1g

50607-30-2
220 suppliers
$13.00/250mg
Yield:50607-30-2 100%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 3 h;Product distribution / selectivity;
Steps:
1
Boc-β-alanine (25 g, 132 mmol), Meldrum's acid (20.9 g, 145 mmol) and 4- dimethylaminopyridine (DMAP, 24.2 g, 198 mmol) were dissolved in 700 mL of dry dichloromethane (DCM) at 00C under nitrogen atmosphere. To this solution 1-(3- dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 30.4 g, 158 mmol) was added. The resulting solution was allowed to reach room temperature and stirred overnight. The reaction mixture was washed (0.5 L x 4) with 5% KHSO4 aqueous solution. The organic layer was dried over anh. Na2SO4, filtered and evaporated under vacuum, affording crude [3-(2,2-dimethyl-4,6-dioxo-[1 ,3]dioxan-5-yl)-3-oxo-propyl]- carbamic acid tert-butyl ester that was dissolved in 600 mL of ethylacetate and refluxed for 4 hours. The solvent was reduced to 150 mL under vacuum and the resulting
References:
NERVIANO MEDICAL SCIENCES S.R.L. WO2008/104475, 2008, A1 Location in patent:Page/Page column 22

1026386-87-7
2 suppliers
inquiry

50607-30-2
220 suppliers
$13.00/250mg

476014-76-3
124 suppliers
$24.00/100mg

50607-30-2
220 suppliers
$13.00/250mg
![Ethyl 3-oxo-3-[(3-ethoxy-3-oxopropyl)aMino]propanoate](/CAS/20150408/GIF/143300-67-8.gif)
143300-67-8
8 suppliers
inquiry

50607-30-2
220 suppliers
$13.00/250mg

90608-67-6
7 suppliers
inquiry

50607-30-2
220 suppliers
$13.00/250mg