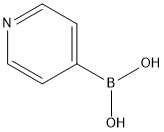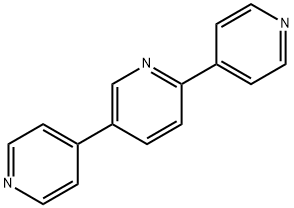
2,5-bis(pyrid-4-yl)pyridine synthesis
- Product Name:2,5-bis(pyrid-4-yl)pyridine
- CAS Number:106047-32-9
- Molecular formula:C15H11N3
- Molecular Weight:233.27
624-28-2
714 suppliers
$5.00/5g
181219-01-2
243 suppliers
$13.00/1g

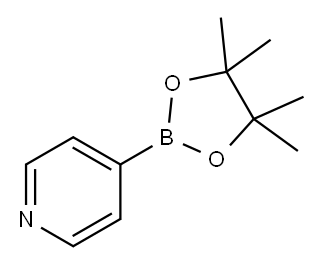
106047-32-9
18 suppliers
inquiry
Yield:106047-32-9 93%
Reaction Conditions:
with potassium phosphate;tris-(dibenzylideneacetone)dipalladium(0);tricyclohexylphosphine tetrafluoroborate in 1,4-dioxane;water at 90; for 8 h;Inert atmosphere;
Steps:
1 Synthesis Scheme (1)
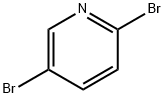
A 100 mL flask was charged with 6.7 18 g (31.6 mmol) of tripotassium phosphate, 24 mL of water, and 30 mL of 1,4-dioxane, and the resulting mixture was bubbled with argon gas, to thereby remove dissolved oxygen. To the resulting solution, 1.500 g (6.33 mmol) of 2,5-diibromopyridine, 5.19 g (25.33 mmol) of4-(4,4,5 ,5-tetramethyl- 1,3 ,2-dioxaborolan-2-yl)pyridine, 0.231 g (0.253 mmol) of tris(dibenzyliden acetone)dipalladium, and 0.224 g (0.607 mmol) of tricyclohexylphosphine tetrafluoroborate were added, and the resulting mixture was stirred for 8 hours at 90°C under argon atmosphere. After the temperature of the mixture was returned to room temperature, the solvent was removed under the reduced pressure, followed by collecting precipitated solids through filtration. The obtained solids were then washed with water. After drying the solids, the dried solids were purified through column chromatography, to thereby obtain colorless solids (yield by weight: 1.393 g, yield: 93%).Subsequently, a 100 mL flask was charged with 1.000 g (4.479 mmol) of the obtained solids, 4.89 g (17.92 mmol) of bromooctyl phosphonate, and 50 mL of DMF (anhydrous), and the resulting mixture was stirred for 12 hours at 90°C under a flow of argon gas. The temperature of the mixture was then returned to room temperature, followed by adding 2-propanol. Thereafter, the precipitated solids were collected through filtration. The solids were purified through recrystallization in methanol, to thereby obtain 1.70 g (yield: 49%) of Electrochromic Compound (Ex.-1) as colorless solids.
References:
WO2016/147543,2016,A1 Location in patent:Paragraph 0093