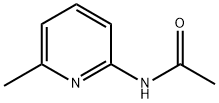
2-ACETAMIDO-6-METHYLPYRIDINE synthesis
- Product Name:2-ACETAMIDO-6-METHYLPYRIDINE
- CAS Number:5327-33-3
- Molecular formula:C8H10N2O
- Molecular Weight:150.18
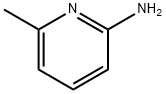
1824-81-3
471 suppliers
$8.00/25g
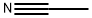
75-05-8
963 suppliers
$10.00/10g

5327-33-3
130 suppliers
$25.00/5g
Yield:5327-33-3 78%
Reaction Conditions:
with phosphoric acid;sodium nitrite at 10 - 20;
Steps:
Synthesis of N-pyridinylacetamides 2a-g upon treatment with a mixture of NaNO2-H3PO4 in a paste with acetonitrile (general procedure).
General procedure: Phosphoric acid (0.7 mL, 12 mmol) was added to acetonitrile (0.5 mL, 9.5 mmol), the resulting mixture was cooled to 10-15 °C. Then, a mixture of aminopyridine 1a-g (2 mmol) and sodium nitrite (0.56 g, 8 mmol) pretriturated in a mortar was added in small portions at such a rate that allowed us to avoid vigorous liberation of nitrogen oxides. The resulting paste was thoroughly triturated and allowed to stand at 10-15 °C for 10 min and then at 20 °C for the period of time indicated in Table 1. The reaction progress was monitored by TLC (eluent hexane-acetone, 1 : 3) and GCMS. Upon completion, the reaction mixture was diluted with water, neutralized with NaHCO3, and extracted with ethyl acetate. The organic layer was separated and dried with Na2SO4, the solvent was evaporated. Compounds 2a-g were purified by recrystallization from ethanol. Yields and m.p. of compounds 2a-g are summarized in Table 1. NMR spectra and m.p. of compounds obtained correspond to the data published for authentic samples.
References:
Chudinov;Dovbnya;Krasnokutskaya;Ogorodnikov;Filimonova [Russian Chemical Bulletin,2016,vol. 65,# 9,p. 2312 - 2314][Izv. Akad. Nauk, Ser. Khim.,2016,# 9,p. 2312 - 2314,3]

1824-81-3
471 suppliers
$8.00/25g

108-24-7
5 suppliers
$14.00/250ML

5327-33-3
130 suppliers
$25.00/5g

1824-81-3
471 suppliers
$8.00/25g

75-36-5
559 suppliers
$17.92/100G

5327-33-3
130 suppliers
$25.00/5g

1824-81-3
471 suppliers
$8.00/25g

64-19-7
1529 suppliers
$10.00/25ML

5327-33-3
130 suppliers
$25.00/5g

1824-81-3
471 suppliers
$8.00/25g

5327-33-3
130 suppliers
$25.00/5g