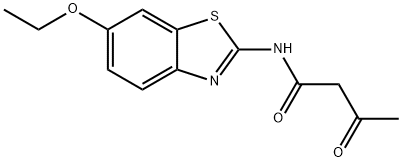
2-ACETOACETAMIDO-6-ETHOXYBENZOTHIAZOLE synthesis
- Product Name:2-ACETOACETAMIDO-6-ETHOXYBENZOTHIAZOLE
- CAS Number:4273-88-5
- Molecular formula:C13H14N2O3S
- Molecular Weight:278.33
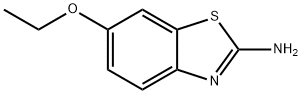
94-45-1
174 suppliers
$11.00/5g
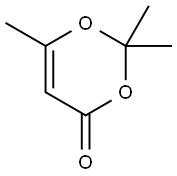
5394-63-8
221 suppliers
$19.00/25g

4273-88-5
16 suppliers
$28.60/100mg
Yield:4273-88-5 41%
Reaction Conditions:
in 5,5-dimethyl-1,3-cyclohexadiene;Reflux;
Steps:
5 General procedure for the synthesis of N-(aryl)-3-oxobutanamides (3a-e)
General procedure: N-(Aryl)-3-oxobutanamides (3a-e) were synthesized accordingto the modified method of Clemens via condensation of 2,2,6-trimethyl-1,3-dioxine-4-one with the appropriate arylamine.50The results are represented in Table 7. 4.2.1.5
N-(6-Ethoxy-2-benzothiazolyl)-3-oxobutanamide (3e)
Pale yellow crystals; Yield 41%; 1H NMR (DMSO-d6) δ (ppm) 11.88 (br s, 1H, NH-amide), 7.62 (d, J = 8.8 Hz, 1H, C4'H-benzothiazole), 7.54 (d, J = 2.4 Hz, 1H, C7'H-benzothiazole), 7.01 (d, J = 8.8 Hz, 1H, C5'H-benzothiazole), 4.06 (q, J = 6.8 Hz, 2H, -OCH2CH3), 3.75 (s, 2H, COCH2CO), 2.23 (s, 3H, CH3CO), 1.35 (t, J = 6.4 Hz, 3H, -OCH2CH3); IR (KBr) ν (cm-1): 3190.2 (N-H, amide), 3085.8 (C-H, aromatic), 2962.5 and 2889.6 (C-H, aliphatic), 1720.6 (C=O, ketone), 1661.9 (C=O, amide); MS m/z (%): 278(58) [M+], 220(18), 194(100), 165(86), 43(41); Anal. Calcd (%) for C13H14N2O3S: C, 56.10; H, 5.07; N, 10.06; S, 11.52. Found: C, 56.23; H, 4.95; N, 10.18; S, 11.41.
References:
Razzaghi-Asl, Nima;Firuzi, Omidreza;Hemmateenejad, Bahram;Javidnia, Katayoun;Edraki, Najmeh;Miri, Ramin [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 22,p. 6893 - 6909]