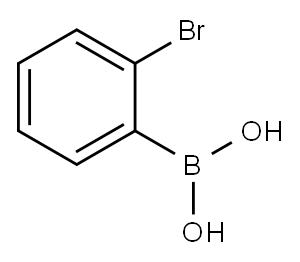
2-Bromophenylboronic acid synthesis
- Product Name:2-Bromophenylboronic acid
- CAS Number:244205-40-1
- Molecular formula:C6H6BBrO2
- Molecular Weight:200.83
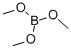
121-43-7
344 suppliers
$14.00/25mL
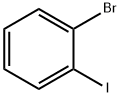
583-55-1
466 suppliers
$7.00/5g

244205-40-1
323 suppliers
$9.00/1g
Yield:244205-40-1 86.83 g
Reaction Conditions:
Stage #1: 1-Bromo-2-iodobenzenewith lithium acetate;1-propylmagnesium chloride;3-butyl-1-methylimidazolium acetate in tetrahydrofuran at 0 - 10; for 4 h;Inert atmosphere;
Stage #2: Trimethyl borate in tetrahydrofuran at 10; for 3 h;Inert atmosphere;
Stage #3: with acetic acid at 15; for 0.333333 h;Inert atmosphere;
Steps:
5.2; 5.3
Step 2: Take 24.9g (0.125mol) of 1-butyl-3methylimidazole acetate, add 4.1g (0.0621mol) of lithium acetate under stirring conditions, and stir until the system is transparent and homogeneous to obtain a catalyst; Weigh 8.605g of the above catalyst, add it to the propylmagnesium chloride solution in tetrahydrofuran in step 1, mix well, and cool to 0°C. Under nitrogen protection and stirring, add 2-bromoiodobenzene solution (a mixed solution of 70.71g (0.25mol) 2-bromoiodobenzene and 110mL tetrahydrofuran) dropwise, and control the dropping rate so that the temperature does not exceed 10°C. Start timing from the dripping of 2-bromoiodobenzene solution and react for 4 hours to obtain 2-bromophenyl magnesium chloride solution; Step 3: Dissolve 249.4 g (2.4 mol) of trimethyl borate in 380 mL of tetrahydrofuran to obtain a trimethyl borate solution; under the protection of nitrogen, add dropwise the 2-bromophenyl magnesium chloride solution prepared in step two, Control the dropping rate so that the temperature does not exceed 10°C, start timing from the dropping of 2-bromophenyl magnesium chloride solution, react for 3 hours, control the temperature at 15°C, drop in acetic acid solution with a mass concentration of 45% by weight, and start timing from the dropping of acetic acid solution. Hydrolysis for 20min,The aqueous phase was extracted twice with 100 mL ethyl acetate, and the extract was washed twice with 200 mL saturated brine. After the solvent was removed under reduced pressure, 150 mL petroleum ether and 150 mL ethyl acetate were added to recrystallize, filtered and dried to obtain a pure white powder. 2-bromophenylboronic acid 86.83g. Based on the molar amount of 2-bromoiodobenzene, the three-step total yield is 86.4%, the HPLC content is 99.84%, the content of 2-iodophenylboronic acid is less than 0.001%, and the content of o-diphenylboronic acid is less than 0.001%.
References:
CN111647011,2020,A Location in patent:Paragraph 0072-0077
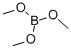
121-43-7
344 suppliers
$14.00/25mL
583-53-9
308 suppliers
$10.00/5g

244205-40-1
323 suppliers
$9.00/1g

583-55-1
466 suppliers
$7.00/5g

244205-40-1
323 suppliers
$9.00/1g

583-53-9
308 suppliers
$10.00/5g

244205-40-1
323 suppliers
$9.00/1g