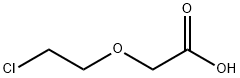
2-CHLOROETHOXY ACETIC ACID synthesis
- Product Name:2-CHLOROETHOXY ACETIC ACID
- CAS Number:14869-41-1
- Molecular formula:C4H7ClO3
- Molecular Weight:138.55
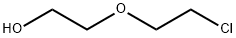
628-89-7
366 suppliers
$14.00/5g

14869-41-1
142 suppliers
$23.00/100mg
Yield:14869-41-1 72%
Reaction Conditions:
Stage #1:2-(2-Chloroethoxy)ethanol with calcium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;sodium hydrogencarbonate in dichloromethane;water at 20; for 0.5 h;
Stage #2: with sodium metabisulfite in dichloromethane;water
Steps:
2 2-(2-chloroethoxy)acetic acid (X)
150 ml dichloromethane, 60 ml tap water, 30.0 ml 2-(2-chloroethoxy)ethanol (XI), 28.52g sodium hydrogencarbonate, and Q.22g TEMPO catalyst are measured to a sulfonating flask equipped with condenser, thermometer, dropping funnel. Then at roo temperature 240.0 ml solution of 55.62g calcium-hypochlorite in tap water is added from the dropping funnel. The mixture is stirred for a further half an hour, then 1.34g sodium metabisulfite is added to the reaction mixture, the two phases are separated in a separation funnel, then the aqueous phase is washed with 2x10 ml dichloromethane. Then the aqueous phase is acidified to pH=T-2 with 34% hydrochloric acid solution. The obtained aqueous solution is extracted with 5x50 ml methyl isobutyl ketone. The combined organic extract is washed with 1x20 ml saturated salt solution, then at a reduced pressure evaporated to constant mass. (0073) The mass of the obtained pale yellow oil : 28.4g, yield 72%.
References:
RICHTER GEDEON NYRT.;SZABÓ, Tamás;NEU, József;GARADNAY, Sándor WO2019/138362, 2019, A1 Location in patent:Page/Page column 9-10
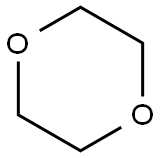
123-91-1
655 suppliers
$16.00/25g

14869-41-1
142 suppliers
$23.00/100mg

17229-14-0
151 suppliers
$25.00/10g

14869-41-1
142 suppliers
$23.00/100mg

31250-08-5
67 suppliers
inquiry

14869-41-1
142 suppliers
$23.00/100mg
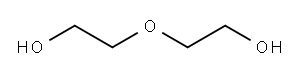
111-46-6
1055 suppliers
$5.00/25g

14869-41-1
142 suppliers
$23.00/100mg