
2-fluoro-1-(3-fluorophenyl)ethanone synthesis
- Product Name:2-fluoro-1-(3-fluorophenyl)ethanone
- CAS Number:1335113-29-5
- Molecular formula:C8H6F2O
- Molecular Weight:156.13

503-20-8
124 suppliers
$40.29/1g
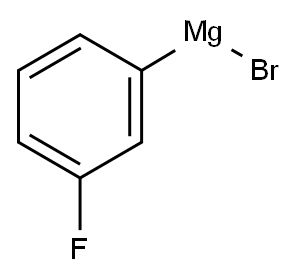
17318-03-5
84 suppliers
$60.00/100mg

1335113-29-5
5 suppliers
inquiry
Yield:1335113-29-5 86%
Reaction Conditions:
Stage #1: fluoroacetonitrile;bromo(3-fluorophenyl)magnesium in diethyl ether at 0;Inert atmosphere;
Stage #2: with hydrogenchloride;water in diethyl ether;Inert atmosphere;
Steps:
General procedure for the preparation of ketones (3,20-21) using basic conditions:
General procedure: The fluorinated nitrile (1 mmol) is dissolved in 5 mL of anhydrous ether. The solution is cooled to 0oC. The organometallic reagent (1.2 mmol; added as solution in ether or hexanes) is added slowly to the reaction mixture under an argon atmosphere. The reaction is stirred for 2 hours and quenched by adding argon-saturated water (5 mL) and 1M HCl (3 mL). To this solution, 50 mL of ethyl acetate is added and the organic layer is washed with water (2 x 20ml), brine (2x 20ml), and then dried over anhydrous sodium sulfate. The product is then isolated by filtration and removal of the solvent. If necessary column chromatography is done with silica gel and hexane:ether mobile phase.
References:
Raja, Erum K.;Klumpp, Douglas A. [Tetrahedron Letters,2011,vol. 52,# 40,p. 5170 - 5172] Location in patent:supporting information; experimental part

226260-01-1
75 suppliers
$15.00/1g

373-53-5
55 suppliers
$165.00/1g

1335113-29-5
5 suppliers
inquiry
2561-17-3
170 suppliers
$6.00/1g

1335113-29-5
5 suppliers
inquiry

53631-18-8
141 suppliers
$15.00/1g

1335113-29-5
5 suppliers
inquiry
1711-07-5
323 suppliers
$6.00/5g

1335113-29-5
5 suppliers
inquiry