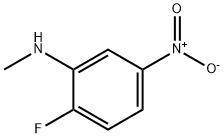
2-Fluoro-N-Methyl-5-nitroaniline synthesis
- Product Name:2-Fluoro-N-Methyl-5-nitroaniline
- CAS Number:110729-51-6
- Molecular formula:C7H7FN2O2
- Molecular Weight:170.14
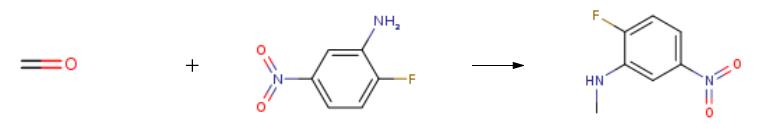
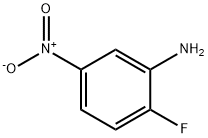
2-fluoro-5-nitroaniline (20.0g, 128.2mmol) and paraformaldehyde (16.0g, 533.3mmol) was dissolved in 500mL of methanol then stirred at room temperature. Then added dropwise NaOMe (3.4g, 63mmol) in methanol 100mL. The reaction was stirred at room temperature for 16 hours then was added NaBH4 (9.7g, 255.2mmol) in two aliquots, stirred for 15 minutes. LCMS traced the reaction. After completion of the reaction was poured into 1M KOH aqueous solution, and stirred to precipitate a solid. Filtered to give a yellow solid 2-fluoro-N-methyl-5-nitroaniline (19.0g, 87% yield).

50-00-0
841 suppliers
$10.00/25g
369-36-8
256 suppliers
$10.00/10g
110729-50-5
0 suppliers
inquiry

110729-51-6
44 suppliers
$30.00/250mg
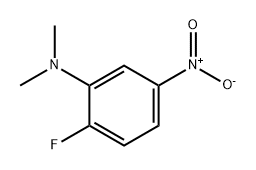
Yield:110729-50-5 88% ,110729-51-6 9%
Reaction Conditions:
with sodium cyanoborohydride;acetic acid in methanol;water at 20; for 18 h;
Steps:
31.A Step A
Commercially available 2-fluoro-5-nitro-aniline (2 g, 12.8 mmol) was dissolved in methanol (100 mL) and a 37% aqueous formaldehyde solution (10 mL, 128 mmol) was added. After the addition of sodium cyanoborohydride (4.2 g, 68 mmol) and acetic acid (15 mL) an exotherm was observed and the reaction mixture was stirred at room remperature for 18 hours. The solvents were evaporated under reduced pressure and the residue was dissolved in dichloromethane (150 mL) and water (50 mL). The pH was adjusted to pH -10/1 1 by the addition of solid sodium hydroxide. The organic phase was separated and the aqueous layer was extracted with dichloromethane (75 mL). The combined organic phase was dried over Na2S04, filtered and the solvent was removed under reduced pressure. The residue was purified by chromatography on HP-Sil SNAP cartridges using a Biotage Isolera Onepurification system employing an ethyl acetate/n-heptane gradient (5/95 -> 30/70) to afford the less polar title compound Frl as a yellow oil (2.09 g, 88 %) and the more polar title compound Frll as a yellow solid (0.2 g, 9 %). Less polar Frl: 1H-NMR (400 MHz, DMSO-d6): δ = 2.88 (s, 6H), 7.36 (dd, 1 H), 7.65 (dd, 1 H), 7.73 (dt, 1 H) More polar Frll: 1H-NMR (400 MHz, DMSO-d6): δ = 2.78 (d, 3H), 6.27-6.31 (br-s, 1 H), 7.26 (dd, 1 H), 7.35 (dd, 1 H), 7.44 (dt, 1 H)
References:
WO2015/110263,2015,A1 Location in patent:Page/Page column 168; 169

50-00-0
841 suppliers
$10.00/25g

369-36-8
256 suppliers
$10.00/10g

110729-51-6
44 suppliers
$30.00/250mg

369-36-8
256 suppliers
$10.00/10g

110729-51-6
44 suppliers
$30.00/250mg