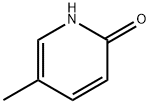
2-Hydroxy-5-methylpyridine synthesis
- Product Name:2-Hydroxy-5-methylpyridine
- CAS Number:1003-68-5
- Molecular formula:C6H7NO
- Molecular Weight:109.13
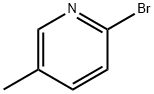
3510-66-5
465 suppliers
$5.00/5g

1003-68-5
381 suppliers
$6.00/1g
Yield:1003-68-5 46%
Reaction Conditions:
Stage #1: 2-Bromo-5-methylpyridinewith potassium tert-butylate in tert-Amyl alcohol at 100; for 40 h;Schlenk technique;Inert atmosphere;
Stage #2: with formic acid at 20; for 24 h;
Steps:
General procedure: In a dry Schlenk tube 2-bromo-6-methylpyridine (5.98 g, 35.0 mmol) was dissolved in 100 mL oft-AmylOH and KOt-Bu (39.3 g , 350.0 mmol) was added. The mixture was stirred at 100 °C for 40 h. The solvent was removed under reduced pressure and the residue was dissolved in 50 mL of HCO2H. The solution was stirred for 24 h at rt, then the pH was set to about 6 using 3N aq. KOH solution. The extraction was performed using CHCl3 (3×) and the combined organic phases were washed with brine, dried over MgSO4, filtered and evaporated. The residue was transferred to a column chromatography (8%MeOH in DCM) to afford 1 (white solid), 2.75 g (72%).
References:
Wysocki, Jedrzej;Schlepphorst, Christoph;Glorius, Frank [Synlett,2015,vol. 26,# 11,art. no. ST-2015-B0171-L,p. 1557 - 1562] Location in patent:supporting information
1603-41-4
542 suppliers
$7.00/25g

1003-68-5
381 suppliers
$6.00/1g
![2,4-Diazabicyclo[4.2.0]oct-7-ene-3,5-dione, 8-methyl-](/CAS/20210305/GIF/72323-49-0.gif)
72323-49-0
0 suppliers
inquiry

1003-68-5
381 suppliers
$6.00/1g

1007-56-3
0 suppliers
inquiry

1003-68-5
381 suppliers
$6.00/1g
108-99-6
324 suppliers
$9.00/5g

1003-68-5
381 suppliers
$6.00/1g