
2-METHYL-2-(4-NITROPHENYL)-2-PROPANOL synthesis
- Product Name:2-METHYL-2-(4-NITROPHENYL)-2-PROPANOL
- CAS Number:22357-57-9
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
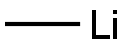
917-54-4
185 suppliers
$44.39/50ml
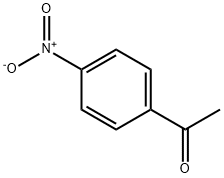
100-19-6
310 suppliers
$5.00/5g

22357-57-9
20 suppliers
$90.00/50mg
Yield: 37%
Reaction Conditions:
Stage #1:methyllithium with titanium tetrachloride in diethyl ether;acetone at -78; for 1.5 h;Inert atmosphere;
Stage #2:(4-nitrophenyl)ethanone in diethyl ether;acetoneInert atmosphere;
Steps:
2-(4-Nitrophenyl)propan-2-ol (18):
TiCl4 (3.02 mL, 27.6 mmol) was added dropwise slowly to dry Et2O (100 mL) in an acetone / dry ice bath (-78 °C). Methyllithium (17.2 mL, 28 mmol, 1.6 M) was then added dropwise slowly to the reaction mixture, which was stirred for 1.5 h. 4-Nitroacetophenone (3.50g, 21.2 mmol) dissolved in Et2O (150 mL) was added dropwise to the reaction mixture, which was stirred for 18 h. The reaction was quenched with water, and the mixture was extracted with CH2Cl2 (3 x 50 mL), which was washed with water and brine, dried over Na2SO4, and evaporated under reduced pressure. Flash chromatography of the crude product using a prepacked 100 g silica column [eluents: solvent A, EtOAc; solvent B, hexanes; gradient, 10% A/90% B (1 CV), 10% A/90% B 60% A/40% B (10 CV), 60% A/40% B (2 CV); flow rate 100.0 mL/min; monitored at 254 and 280 nm] yielded 2-(4-nitrophenyl)propan-2-ol (18) (1.42 g, 7.84 mmol, 37%) as an orange oil.1H NMR (600 MHz, CDCl3) δ 8.16 (2H, d, J = 8.9 Hz), 7.65 (2H, d, J = 8.9 Hz), 1.61 (7H, s).13C NMR (151 MHz, CDCl3) δ 156.52, 146.64, 125.51, 123.45, 72.49, 31.69.
References:
Winn, Blake A.;Shi, Zhe;Carlson, Graham J.;Wang, Yifan;Nguyen, Benson L.;Kelly, Evan M.;Ross, R. David;Hamel, Ernest;Chaplin, David J.;Trawick, Mary L.;Pinney, Kevin G. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 3,p. 636 - 641] Location in patent:supporting information
1817-47-6
120 suppliers
$21.00/1g

22357-57-9
20 suppliers
$90.00/50mg

1817-47-6
120 suppliers
$21.00/1g

22357-57-9
20 suppliers
$90.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

100-19-6
310 suppliers
$5.00/5g

1817-47-6
120 suppliers
$21.00/1g

22357-57-9
20 suppliers
$90.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
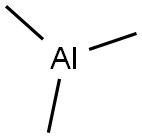
75-24-1
166 suppliers
$54.50/100ml

100-19-6
310 suppliers
$5.00/5g

22357-57-9
20 suppliers
$90.00/50mg