
2-Methylbenzo[g]quinazolin-4(3H)-one synthesis
- Product Name:2-Methylbenzo[g]quinazolin-4(3H)-one
- CAS Number:16673-88-4
- Molecular formula:C13H10N2O
- Molecular Weight:210.23
Yield:16673-88-4 72%
Reaction Conditions:
Stage #1: acetic anhydride;2-Amino-3-naphthoic acid for 4 h;Reflux;
Stage #2: with ammonium hydroxide at 100; for 12 h;
Steps:
2 General Method A:
General procedure: 2-Amino-3-naphthoic acid or 2-amino-3-benzoic acid (1 eq) was dissolved in acetic anhydride (0.2 M final) and heated to reflux. After stirring a reflux for 4 h, reaction mixture was cooled to room temperature and solvent was removed by rotary evaporator. Concentrated NH4OH (0.2 M final) was added to residue and the solution was heated at 100 °C for 12 h. Once cooled to room temperature, the resulting precipitate was collected by filtration and washed with H20 and methanol yielding a solid (1.72 g, 72%). 'H and 13C NMR were consistent with previous reports.
References:
WO2019/177975,2019,A1 Location in patent:Page/Page column 83; 84-85
![4H-Naphth[2,3-d][1,3]oxazin-4-one, 2-methyl-](/CAS/20210305/GIF/16673-86-2.gif)
16673-86-2
0 suppliers
inquiry
![2-Methylbenzo[g]quinazolin-4(3H)-one](/CAS/GIF/16673-88-4.gif)
16673-88-4
10 suppliers
inquiry

19717-59-0
14 suppliers
inquiry
![2-Methylbenzo[g]quinazolin-4(3H)-one](/CAS/GIF/16673-88-4.gif)
16673-88-4
10 suppliers
inquiry

79979-69-4
28 suppliers
$489.00/1g
![2-Methylbenzo[g]quinazolin-4(3H)-one](/CAS/GIF/16673-88-4.gif)
16673-88-4
10 suppliers
inquiry
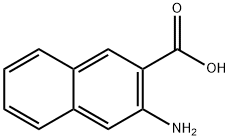
5959-52-4
206 suppliers
$44.31/1g
![2-Methylbenzo[g]quinazolin-4(3H)-one](/CAS/GIF/16673-88-4.gif)
16673-88-4
10 suppliers
inquiry
