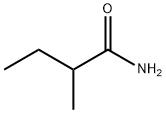
2-methylbutyramide synthesis
- Product Name:2-methylbutyramide
- CAS Number:1113-57-1
- Molecular formula:C5H11NO
- Molecular Weight:101.15
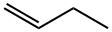
106-98-9
159 suppliers
$10.00/25 g
201230-82-2
1 suppliers
inquiry

1113-57-1
5 suppliers
inquiry
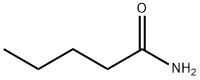
626-97-1
119 suppliers
$20.00/1g
Yield:-
Reaction Conditions:
with ammonium chloride in 1-methyl-pyrrolidin-2-one at 129.84; under 22502.3 Torr; for 24 h;Catalytic behavior;Overall yield = 55percent;
Steps:
2.4. Typical procedure for hydroaminocarbonylation reaction
General procedure: The hydroaminocarbonylation reaction was carried out using astainless steel autoclave reactor (30 mL inner volume with a poly tetrafluoroethylene lining). Ammonium salt (1 mmol), PdPOPs-PPh3(0.0072 g, Pd was 0.7 wt%), and NMP (N-Methyl pyrrolidone, 4 g) weresuccessively loaded into the reactor. The reactor was purged three timesby N2. Then, the reactor was charged with the mixed gas (C2H4/CO =1:5) up to 3 MPa at room temperature. The reactor was heated by anelectric heating jacket to 403 K and stirred for 24 h. After the reaction,the reactor was cooled down to room temperature using a water bath.The yield was determined by the GC analysis on an Agilent 7890Aequipped with a capillary column (HP-5 column, 30 m ×0.32 μmdiameter) using a flame ionization detector. Ethanol was used as aninternal standard for the GC analysis
References:
Sun, Zhao;Yan, Li;Ji, Guangjun;Wang, Guoqing;Ma, Lei;Jiang, Miao;Li, Cunyao;Ding, Yunjie [Applied Catalysis A: General,2021,vol. 614,art. no. 118026]

16668-14-7
0 suppliers
inquiry

1113-57-1
5 suppliers
inquiry

18936-17-9
77 suppliers
$34.00/1kg

1113-57-1
5 suppliers
inquiry