
2,3,4,5-Tetrahydro-7,8-dinitro-3-(trifluoroacetyl)-1,5-methano-1H-3-benzazepine synthesis
- Product Name:2,3,4,5-Tetrahydro-7,8-dinitro-3-(trifluoroacetyl)-1,5-methano-1H-3-benzazepine
- CAS Number:230615-59-5
- Molecular formula:C13H10F3N3O5
- Molecular Weight:345.23
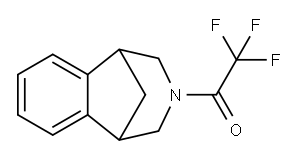
230615-51-7
104 suppliers
$12.00/250mg

230615-59-5
145 suppliers
inquiry
Yield:230615-59-5 68.9%
Reaction Conditions:
with trifluorormethanesulfonic acid;nitric acid in dichloromethane at 0 - 30;Product distribution / selectivity;
Steps:
10
Example 10Procedure for Preparation of 1-(4,5-Dinitro-10-aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-triene-10-yl)-2,2,2-trifluoro-ethanone (IX); Fuming nitric acid (390.2 g) was added over 25 to 35 minutes at 0° to 5° C. to a solution of tri-fluoro methane sulfonic acid (1.7 kg) in MDC (2.52 l). The mixture was stirred for 10-15 minutes. To this resulting organic layer, 1-(10-Aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-triene-10-yl)-2,2,2-trifluoro-ethanone in MDC was added over 1.0 to 1.5 hours at 0° to 5° C. After completion of addition, the temperature was immediately raised to 25° to 30° C. The reaction mass was stirred at 25° to 30° C. for 2.0 hours. The progress of the reaction was checked by HPLC. The reaction mixture was quenched in DM water (6.5 l) at 0° to 5° C. The layers were separated, and the aqueous layer was extracted with MDC (2×1.26 l). The combined organic layer was washed with DM water (3×3.2 l), and then with an 8 percent aqueous NaHCO3 solution (1×3.2 l) and DM water (1×2.5 l). The organic layer was concentrated to provide crude 1-(4,5-Dinitro-10-aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-triene-10-yl)-2,2,2-trifluoro-ethanone. This was triturated with ethyl acetate (1.30 l) at 55° to 60° C. for 2 hours. The solid was filtered and washed with chilled ethyl acetate (630 ml) to provide pure 1-(4,5-Dinitro-10-aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-triene-10-yl)-2,2,2-trifluoro-ethanone having a yield of 68.9 percent and an HPLC purity of 99.55 percent.
References:
US2009/318695,2009,A1 Location in patent:Page/Page column 9-10