
3-(1-Naphthoyl)indole synthesis
- Product Name:3-(1-Naphthoyl)indole
- CAS Number:109555-87-5
- Molecular formula:C19H13NO
- Molecular Weight:271.31
120-72-9
610 suppliers
$6.00/25g
879-18-5
270 suppliers
$6.00/5g

109555-87-5
109 suppliers
$61.00/5mg
Yield:109555-87-5 93%
Reaction Conditions:
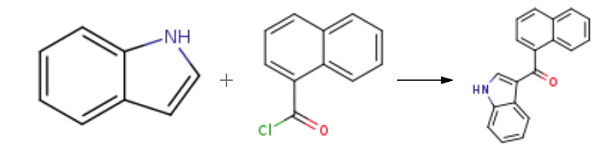
with diethylaluminium chloride in toluene at 0 - 20; for 24 h;Friedel-Crafts Acylation;
Steps:
2.1 Synthesis of synthetic cannabinoids
General procedure: JWH-073 and JWH-018 were synthesized according to previous reports (Blaazer et al., 2011) in high yield (Fig.1). The intermediate (1H-indol-3-yl) (naphthalene-1-yl)methanone (2) was prepared by Friedel-Crafts acylation of indole (1) with 1-naphthoyl chloride in the presence of diethylaluminium chloride in toluene. In order to obtain (2) in high yield, the optimal reaction conditions were performed with different molar ratios of 1-naphthoyl chloride, indole and amount of diethylaluminum chloride and purification was achieved via column chromatography or recrystallization. Each of the final compounds was purified using hexane and ethyl acetate (95:5, v/v) by column chromatography to give JWH-073 and JWH-018 as an oily yellow gum. For the intermediate, a molar ratio of 1.2-1.4 times of indole to 1-naphthoyl chloride and diethylaluminium chloride achieved a yield of 85-93%. Subsequent N-alkylation with 1-bromobutane and 1-bromopentane using potassium hydroxide and dimethylformamide in acetone provided JWH-073 and JWH-018.
References:
Cooper, Ziva D.;Poklis, Justin L.;Liu, Fei [Neuropharmacology,2018,vol. 134,p. 92 - 100]

120-72-9
610 suppliers
$6.00/25g
2122-70-5
124 suppliers
$26.00/5g

109555-87-5
109 suppliers
$61.00/5mg

120-72-9
610 suppliers
$6.00/25g

109555-87-5
109 suppliers
$61.00/5mg

576-15-8
91 suppliers
$50.00/1g

20300-10-1
20 suppliers
$35.20/1g

109555-87-5
109 suppliers
$61.00/5mg

62615-78-5
1 suppliers
inquiry

20300-10-1
20 suppliers
$35.20/1g

109555-87-5
109 suppliers
$61.00/5mg