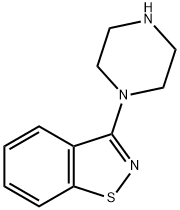
3-(1-Piperazinyl)-1,2-benzisothiazole synthesis
- Product Name:3-(1-Piperazinyl)-1,2-benzisothiazole
- CAS Number:87691-87-0
- Molecular formula:C11H13N3S
- Molecular Weight:219.31
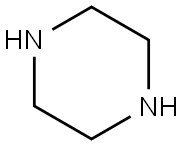
110-85-0
550 suppliers
$5.00/5 g
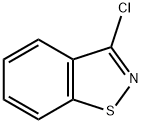
7716-66-7
273 suppliers
$32.00/5g

87691-87-0
399 suppliers
$10.00/1g
Yield:87691-87-0 85%
Reaction Conditions:
in ethanol at 80; for 36 h;
Steps:
4.1.17. General procedure for synthesis of 3-(piperazin-1-yl)benzo[d]isothiazole
General procedure for synthesis of 3-(piperazin-1-yl)benzo[d]isothiazole
The compound 3-chloro-1, 2-benzisothiazole (1 mmol) was allowed to react with piperazine (1.2 mmol) in Ethanol at 80 °C for 36 h.
Then reaction mixture was concentrate and RM was dissolved in ethyl acetate and washed with water.
Ethylacetate layer dried with Na2SO4 and concentrate them and get 3-(piperazin-1-yl)benzo[d]isothiazole.
White solid, 85% yield, mp 215-217 °C, 1H NMR (400 MHz, CDCl3): δ = 7.42 (t, J = 7.2 Hz, 1H), 7.29 (m, 3H), 4.14 (t, J = 4.8 Hz, 4H), 3.15 (t, J = 4.8 Hz, 4H); 13C NMR (100 MHz, DMSO+CDCl3): 166.8, 156.2, 135.8, 128.5, 127.6, 125.2, 124.9, 119.8, 53.6, 49.2, 46.6, 45.1, 7.1. MS calcd for C19H20N4OS2: 219.31. Found: 220.33, (M+); Anal. Calcd for C19H20N4OS2: C, 60.24; H, 5.97; N, 19.16; S, 14.62; Found: C, 60.25; H, 5.99; N, 19.13; S, 14.61.
References:
Reddy, Kummetha Indrasena;Srihari, Konduri;Renuka, Janupally;Sree, Komanduri Shruthi;Chuppala, Aruna;Jeankumar, Variam Ullas;Sridevi, Jonnalagadda Padma;Babu, Kondra Sudhakar;Yogeeswari, Perumal;Sriram, Dharmarajan [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 23,p. 6552 - 6563]

1384511-83-4
0 suppliers
inquiry

87691-87-0
399 suppliers
$10.00/1g

110-85-0
550 suppliers
$5.00/5 g

7716-66-7
273 suppliers
$32.00/5g

87691-87-0
399 suppliers
$10.00/1g
87691-88-1
181 suppliers
$9.00/1g

19602-82-5
19 suppliers
$144.00/1mg

87691-87-0
399 suppliers
$10.00/1g
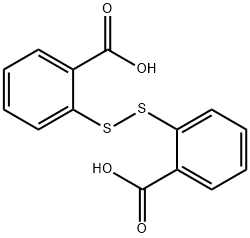
119-80-2
420 suppliers
$7.00/25g

87691-87-0
399 suppliers
$10.00/1g