
3-(3-METHOXY-PHENYL)-THIOPHENE synthesis
- Product Name:3-(3-METHOXY-PHENYL)-THIOPHENE
- CAS Number:92575-93-4
- Molecular formula:C11H10OS
- Molecular Weight:190.26
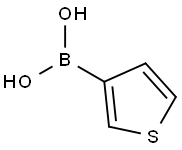
6165-69-1
343 suppliers
$12.72/1gm:
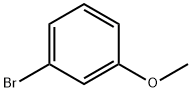
2398-37-0
569 suppliers
$8.00/10g

92575-93-4
11 suppliers
inquiry
Yield:92575-93-4 66%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,4-dioxane;water at 100; for 1 h;Inert atmosphere;
Steps:
18.1 Step 1) Synthesis of 3-(3-methoxyphenyl)thiophene
3-Methoxybromobenzene (5.00 g, 26.70 mol), 3-thiopheneboronic acid (5.13 g, 40.10 mmol), tetratriphenylphosphinepalladium (3.09 g, 2.67 mmol) and potassium carbonate (11.11 g, 80.38 mmol) were added into a reactionflask. 1,4-Dioxane (100 mL) and water (10 mL) were then added. The resulting mixture was heated to 100°C under theprotection of nitrogen and reacted overnight. The reaction solution was cooled to rt and filtered through a celite pad. Thefilter cake was washed with ethyl acetate (20 mL), then the filtrate was concentrated and the residue was purified bysilica gel column chromatography (PE) to give the title compound as light yellow oil (3.36g, 66%).1H NMR (400 MHz, CDCl3) δ (ppm):7.89 (dd, J = 2.7, 1.1 Hz, 1H), 7.63 (dd, J = 5.0, 2.9 Hz, 1H), 7.57 (dd, J = 5.0, 1.1Hz, 1H), 7.35 - 7.26 (m, 3H), 6.90 - 6.84 (m, 1H), 3.81 (s, 3H).
References:
EP3686201,2020,A1 Location in patent:Paragraph 0343-0344

6165-69-1
343 suppliers
$12.72/1gm:
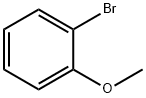
578-57-4
308 suppliers
$6.00/10g

92575-93-4
11 suppliers
inquiry

1711-05-3
172 suppliers
$8.00/1g

92575-93-4
11 suppliers
inquiry
