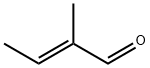
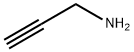
3,4,5-trimethylpyridine synthesis
- Product Name:3,4,5-trimethylpyridine
- CAS Number:20579-43-5
- Molecular formula:C8H11N
- Molecular Weight:121.18
Yield:20579-43-5 89%
Reaction Conditions:
with sodium hydrogencarbonate in N,N-dimethyl-formamide at 20 - 80; for 15 h;Molecular sieve;Inert atmosphere;
Steps:
3 General Method of Preparation of Substituted Pyridines
General procedure: To a stirred solution of α, β-unsaturated carbonyl compounds 1a-s (0.6 mmol) and propargylic amine/propargylic amine hydrochloride (0.9 mmol) in DMF (3 mL) were added 4 molecular sieves (200 mg) and NaHCO3 (1.2 mmol for free propargylic amines and 1.8 mmol for propargylic amine hydrochlorides) at room temperature under an argon atmosphere. The reaction mixture was stirred for 3 h at room temperature, followed by 12 h at 80° C. The mixture was filtered through Celite, washed with Et2O (10 mL) and water (10 mL) was added to the filtrate. The two layers were separated, and the aqueous layer was extracted with Et2O (2×10 mL). The combined organic layer was washed with ice cold water (2×15 mL), dried over magnesium sulfate and evaporated under reduced pressure. The crude product was purified by flash column chromatography on silica gel to afford the corresponding pyridines. Glassware was dried in an oven (120° C.), heated under reduced pressure, and cooled under argon before use. Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. Reactions were monitored by thin-layer chromatography on Analtech silica gel plates using UV-light and ceric sulfate or β-naphthol for visualization. Column chromatography was performed on silica gel (230-400 mesh) using n-hexane/ethyl acetate, diethyl ether/hexanes as eluents. Evaporation of solvents was conducted under reduced pressure at 50° C. FTIR spectra were recorded neat on a Perkin-Elmer Spectrum 65. NMR spectra were recorded on a Bruker Avance III 400 NMR spectrometer at 400 MHz (1H) and 100 MHz (13C), respectively. Deuterated chloroform was used as the solvent unless otherwise noted, and spectra were calibrated against the residual solvent peak (7.24 ppm for 1H and 77.0 ppm for 13C). Chemical shifts (δ) and coupling constants (J) are given in ppm (parts per million) and Hz (Hertz), respectively. The following abbreviations were used to explain multiplicities: s=singlet, d=doublet, t=triplet, m=multiplet, bs=broad singlet. High Resolution mass spectra were obtained on a VG 70-70H or LC/MSD trapSL spectrometer operating at 70 eV using a direct inlet system.
References:
US2020/95245,2020,A1 Location in patent:Paragraph 0195; 0196; 0197

3430-23-7
190 suppliers
$5.00/250mg
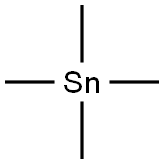
594-27-4
113 suppliers
$31.00/5g

20579-43-5
14 suppliers
inquiry
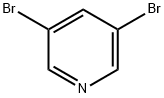
625-92-3
478 suppliers
$6.00/10g

20579-43-5
14 suppliers
inquiry

98593-42-1
0 suppliers
inquiry

20579-43-5
14 suppliers
inquiry

98490-98-3
0 suppliers
inquiry

20579-43-5
14 suppliers
inquiry