
3,5-DiMethylMorpholine synthesis
- Product Name:3,5-DiMethylMorpholine
- CAS Number:123-57-9
- Molecular formula:C6H13NO
- Molecular Weight:115.17
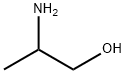
6168-72-5
282 suppliers
$11.92/25g
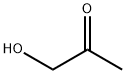
116-09-6
250 suppliers
$11.00/25g

123-57-9
46 suppliers
$140.00/50 mg
Yield:-
Reaction Conditions:
Stage #1:2-Amino-1-propanol;hydroxy-2-propanone with hydrogen;platinum(IV) oxide in ethanol at 20; under 1991.07 Torr;
Stage #2: with sulfuric acid at 180; for 8 h;
Stage #3: with potassium hydroxide in water at 20;
Steps:
11.1
Acetol (10.9 g, 0.133 mmol, 1.17 eq) and [DL-2-AMINO-1-PROPANOL] (8.5 g, 0.113, 1.0 eq) are combined in 200 mL of ethanol. The atmosphere above the solvent is purged with nitrogen, platinum oxide (50 mg, 85%, Englehart) is added and the mixture is hydrogenated at room temperature at 38.5 psi overnight. An additional 50 mg of platinum oxide catalyst is added and the reaction mixture is hydrogenated for an additional 18 h. The reaction mixture is filtered through a pad of solka-floc, rinsed thoroughly with ethanol and the filtrate is concentrated. The residue is purified by silica gel chromatography using chloroform-methanol-ammonium hydroxide (29%) (85: 15: 1) as the eluent to give 10.67 g [(71%)] of the aminodiol. Subsequent dehydration of the aminodiol (10.1 g, 0.0759 mol) is carried out in a flask having ample void volume to accommodate the frothing generated by heating with concentrated sulfuric acid (14.14 g, 0.144 mol, 1.90 eq) at [180°C] for 8 h. The black mixture is cooled in an ice bath while potassium hydroxide (17.1 g, 0.304 mol, 4.0 eq) in 85 mL of water is added dropwise over a period of 25 min. The basic suspension is stirred at room temperature overnight, is filtered through a pad of celite and the pad is rinsed two times with water. The aqueous filtrate is extracted five times with chloroform-methanol (85: 15), dried [(NAS04)] and is concentrated on a rotary evaporator, keeping the water bath temperature < 25°C to minimize loss of volatile product 7.19 g (82%) as a colorless liquid [: 1H] NMR (400 MHz, [CDC13)] [8] 3.62, 3.23, 3.20, 3.07, 2.89, 1.03, 0. 88 3.
References:
PHARMACIA & UPJOHN COMPANY WO2004/31195, 2004, A1 Location in patent:Page 44
2294-46-4
19 suppliers
inquiry

123-57-9
46 suppliers
$140.00/50 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

123-57-9
46 suppliers
$140.00/50 mg
1194-02-1
501 suppliers
$6.00/10g