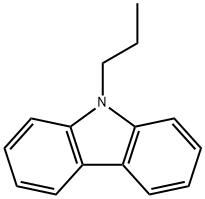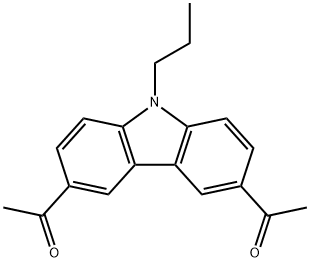
3,6-Diacetyl-9-propyl-9H-carbazole synthesis
- Product Name:3,6-Diacetyl-9-propyl-9H-carbazole
- CAS Number:1483-96-1
- Molecular formula:C19H19NO2
- Molecular Weight:293.36
Yield:1483-96-1 85%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 20; for 50 h;Cooling with ice;Friedel-Crafts Acylation;
Steps:
General Synthetic Procedure of 3,6-Diacetyl-9-alkyl Carbazole (2a-c)
General procedure: Aluminium chloride powder (1.10 g, 8 mmol) was dissolved partly in dichloromethane (10 mL) in a 100 mL three-necked flask with constant stirring until the solution became orange-yellow. To the solution acetyl chloride (1 mL, 10 mmol) in dichloromethane and compound 1 (2 mmol) in dichloromethane was added dropwise to it in an ice bath. The reaction was carried out in the ice bath for 2 h and further stirred at room temperature for 48h. The reaction mixture was then poured into ice water (400 mL) with vigorous stirring to get a pale-yellow precipitate. The crude products were filtered, washed with water, dried, and then recrystallized from alcohol to get compound 2.13
References:
Wu, Limin;Wu, Panliang;Guo, Dongcai;Fu, Wenqiang;Li, Dong;Luo, Tao [Croatica Chemica Acta,2015,vol. 88,# 1,p. 1 - 6]
86-74-8
320 suppliers
$10.00/5g

1483-96-1
0 suppliers
inquiry