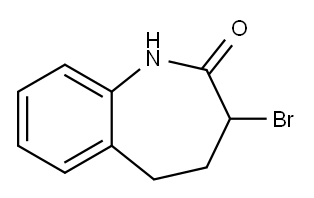
3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one synthesis
- Product Name:3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one
- CAS Number:86499-96-9
- Molecular formula:C10H10BrNO
- Molecular Weight:240.1

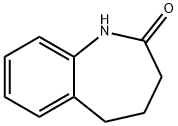
4424-80-0
141 suppliers
$25.00/1g
![3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one](/CAS/GIF/86499-96-9.gif)
86499-96-9
269 suppliers
$14.00/1g
Yield:86499-96-9 70.1%
Reaction Conditions:
Stage #1:2,3,4,5-tetrahydro-1H-1-benzo[b]azepin-2-one with phosphorus pentachloride;iodine in chloroform at 0; for 0.5 h;
Stage #2: with bromine in chloroform at 0 - 20; for 4 h;Refluxed;
Steps:
4.A(2)
A solution of Part A(1) compound (1.0 g, 6.20 mmoles) in dry CHCl3 (15 ml) was cooled down to 0° C. (ice-salt bath), treated with PCl5 (1.5 g, 7.20 mmoles) followed by I2 (15 mg) then stirred at 0° C. under argon for 30 minutes. The yellow solution was treated with Br2 (0.39 ml or 1.2 g, 7.51 mmoles), warmed up to room temperature and refluxed under argon for 4.0 hours. The mixture was then poured into ice-water (20 g), stirred and the phases were separated, washing the aqueous phase with CHCl3 (25 ml). The combined organic extracts were washed with H2O (5.0 ml), dried (anhydrous MgSO4), filtered, evaporated to dryness and dried in vacuo. The crude product mixture was chromatographed on a silica gel column (Merck, 70 g), eluting the column with EtOAc:Hexane (1:9) to give title compound as off-white precipitates (1.137 g., m.pt. 170-172°, 70.1%) with consistent 1H-NMR and 13C-NMR spectral data. [00180] TLC: Rf 0.13 (Silica gel; EtOAc:Hexane-1:4; UV).
References:
Bristol-Myers Squibb Company US6777550, 2004, B1 Location in patent:Page column 28

56384-59-9
1 suppliers
inquiry
![3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one](/CAS/GIF/86499-96-9.gif)
86499-96-9
269 suppliers
$14.00/1g
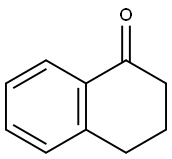
529-34-0
482 suppliers
$6.00/10g
![3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one](/CAS/GIF/86499-96-9.gif)
86499-96-9
269 suppliers
$14.00/1g

1056246-70-8
1 suppliers
inquiry
![3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one](/CAS/GIF/86499-96-9.gif)
86499-96-9
269 suppliers
$14.00/1g
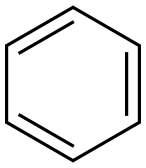
71-43-2
671 suppliers
$11.19/25ML
![3-Bromo-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one](/CAS/GIF/86499-96-9.gif)
86499-96-9
269 suppliers
$14.00/1g